Characterization of the conformational probability of N-acetyl-phenylalanyl-NH2 by RHF, DFT, and MP2 computation and AIM analyses, confirmed by jet-cooled infrared data.
Gregory A Chass, Rei S Mirasol, David H Setiadi, Ting-Hua Tang, Wutharath Chin, Michel Mons, Iliana Dimicoli, Jean-Pierre Dognon, Bela Viskolcz, Sandor Lovas, Botond Penke, Imre G Csizmadia
Index: J. Phys. Chem. A 109(24) , 5289-302, (2005)
Full Text: HTML
Abstract
Computational and experimental determinations were carried out in parallel on the conformational probability of N-Acetyl-Phenylalanine-NH2 (NAPA). Ab initio computations were completed at the BLYP/6-311G(df,p), B3LYP/6-31G(d), B3LYP/6-31G(d,p), and B3LYP/6-31+G(d) levels of theory, labeled L/61fp, B/6, B/6p, and B/6+, respectively. Three experimentally identified conformers were compared with theoretical data, confirming their identities as the betaLanti, gammaLgauche+, and gammaLgauche- (BACKBONESIDECHAIN) conformers. Evidence comes from matching experimental and theoretical data for all three constituent N-H stretches of NAPA, with a Delta(Experimental-Theoretical) = approximately 1-3 cm(-1), approximately 0-5 cm(-1), and approximately 1-6 cm(-1), at the L/61fp and B/6+ levels, respectively. Corrected-ZPE relative energies were computed to be 0.14, 0.00, 0.26 and 0.00, 0.67, 0.57 (kcal*mol(-1)) for the betaLanti, gammaLgauche+, and gamma(Lgauche- conformers, respectively, at the L/61fp and B/6+ levels, respectively. The MP2/6-31+G(d) level of theory was subsequently found to give similar relative energies. Characterization of the intramolecular interactions responsible for red and blue shifting of the N-H stretches showed the existence of the following intramolecular interactions: C=O[i]- - -HN[i], (Ar[i])-Cgamma- - -HN[i+1], (Ar[i])-Cdelta-H- - -O=C[i-1] for betaLanti; C=O[i-1]- - -HN[i+1], (Ar[i])-Cgamma- - -HN[i+1], (Ar[i])-C-H- - -O=C[i] for gammaLgauche+; and C=O[i-1]- - -HN[i+1] for gammaLgauche-. Each of these interactions were further investigated and subsequently characterized by orbital population and Atoms-In-Molecules (AIM) analyses, with the identity of overlap and bond critical points (BCP) serving as 'scoring criteria', respectively. Experimental and theoretical carbonyl stretches were also compared and showed good agreement, adding further strength to the synergy between experiment and theory.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
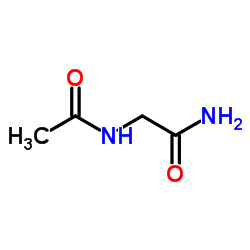 |
N2-Acetylglycinamide
CAS:2620-63-5 |
C4H8N2O2 |
|
[The systems analysis of the effect of piracetam, glycine an...
1993-01-01 [Eksp. Klin. Farmakol. 56(4) , 55-7, (1993)] |
|
[Comparative influence of nootropic preparations on the emet...
1989-06-01 [Biull. Eksp. Biol. Med. 107(6) , 711-3, (1989)] |
|
Additive transfer free energies of the peptide backbone unit...
2004-02-10 [Biochemistry 43(5) , 1329-42, (2004)] |
|
Formation and stability of peptide enolates in aqueous solut...
2002-07-17 [J. Am. Chem. Soc. 124(28) , 8251-9, (2002)] |
|
Preserved acetazolamide reactivity in lacunar patients with ...
2012-05-01 [J. Cereb. Blood Flow Metab. 32(5) , 844-50, (2012)] |