In vitro properties of surface-modified solid lipid microspheres containing an antimalarial drug: halofantrine.
Anthony A Attama, Collins N Igbonekwu
Index: Asian Pac. J. Trop. Med. 4(4) , 253-8, (2011)
Full Text: HTML
Abstract
To formulate and evaluate in vitro, surface-modified solid lipid microspheres containing halofantrine using lipid matrix formed from goat fat and a phospholipid (P90H).The model drug, halofantrine in an increasing concentration of 1%, 2%, 3%, 4% and 5% w/w was incorporated into surface-modified solid lipid microspheres formulated by hot homogenization. Effect of drug concentration on the encapsulation efficiency was studied. The dispersion was evaluated using particle size, particle morphology, pH and encapsulation efficiency. The drug formulation with highest encapsulation efficiency was selected and used for the release studies and compared with the release from a commercial dosage form (Halfan® 250 mg tablet, Glaxo-Smithkline, Mayenne France) using simulated gastric fluid (SGF pH 1.2), simulated intestinal fluid (SIF pH 7.2) and phosphate buffer (pH 6.8) as biorelevant media. Results were analyzed statistically and the level of significance was taken to be P < 0.05).Discrete and spherical solid lipid microspheres were produced. The particle size of the dispersion was low (32.48-33.87 μm) with minimal particle growth and high encapsulation efficiencies (86.8%-91.0%) after 3 months. The pH of the microspheres dispersion changed appreciably after 3 months. In vitro release result obtained revealed sustained and controlled drug release from the lipid microspheres compared with the tablet dosage form.Formulation of halofantrine as solid lipid microspheres presents a better alternative to the conventional tablet formulation as the in vitro dissolution of the highly lipophilic halofantrine was highly improved.Copyright © 2011 Hainan Medical College. Published by Elsevier B.V. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
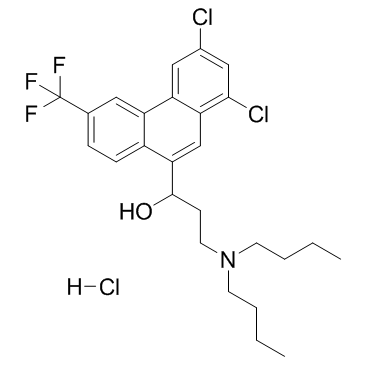 |
Halofantrine hydrochloride
CAS:36167-63-2 |
C26H31Cl3F3NO |
|
Effect of serum lipoproteins on stereoselective halofantrine...
2012-07-01 [Chirality 24(7) , 558-65, (2012)] |
|
Discordant temporal evolution of Pfcrt and Pfmdr1 genotypes ...
2012-03-01 [Antimicrob. Agents Chemother. 56(3) , 1382-9, (2012)] |
|
Using resin to generate a non-invasive intestinal bile-deple...
2012-09-29 [Eur. J. Pharm. Sci. 47(2) , 347-51, (2012)] |
|
Population pharmacokinetics of halofantrine in healthy volun...
2012-11-01 [J. Pharm. Pharmacol. 64(11) , 1603-13, (2012)] |
|
CDA: combinatorial drug discovery using transcriptional resp...
2012-01-01 [PLoS ONE 7(8) , e42573, (2012)] |