Journal of the American Chemical Society
2012-03-21
Identification of the product of toxoflavin lyase: degradation via a Baeyer-Villiger oxidation.
Benjamin Philmus, Sameh Abdelwahed, Howard J Williams, Michael K Fenwick, Steven E Ealick, Tadhg P Begley
Index: J. Am. Chem. Soc. 134(11) , 5326-30, (2012)
Full Text: HTML
Abstract
Toxoflavin (an azapteridine) is degraded to a single product by toxoflavin lyase (TflA) in a reaction dependent on reductant, Mn(II), and oxygen. The isolated product was fully characterized by NMR and MS and was identified as a triazine in which the pyrimidine ring was oxidatively degraded. A mechanism for toxoflavin degradation based on the identification of the enzymatic product and the recently determined crystal structure of toxoflavin lyase is proposed.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
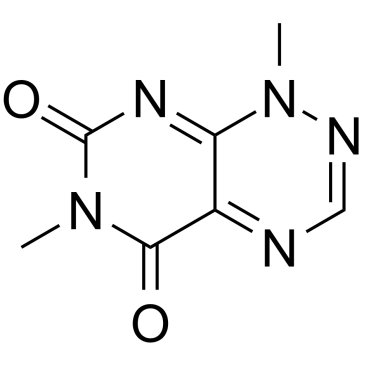 |
Toxoflavin
CAS:84-82-2 |
C7H7N5O2 |
Related Articles:
More...
|
Investigations into the Biosynthesis, Regulation, and Self-R...
2015-08-17 [ChemBioChem. 16 , 1782-90, (2015)] |
|
The quorum sensing-dependent gene katG of Burkholderia gluma...
2009-07-01 [J. Bacteriol. 191(13) , 4152-7, (2009)] |
|
[The stacking interaction of antibiotics of the pyrimido[5,4...
1994-01-01 [Izv. Akad. Nauk Ser. Biol. (1) , 145-7, (1994)] |
|
Structural and functional analysis of phytotoxin toxoflavin-...
2011-01-01 [PLoS ONE 6(7) , e22443, (2011)] |
|
Simultaneous determination of toxic metabolites by linear co...
1991-09-01 [Analyst 116(9) , 919-22, (1991)] |