| Structure | Name/CAS No. | Articles |
|---|---|---|
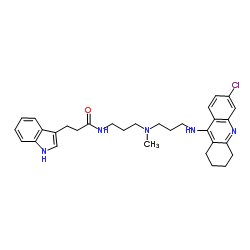 |
3-Hydroxybutyrate dehydrogenase
CAS:9028-38-0 |
|
 |
SepharoseCL-6B
CAS:62610-50-8 |