Research spotlight: Microwave chemistry enabling the synthesis of biologically relevant amines.
John Spencer
Index: Future Med. Chem. 2(2) , 161-8, (2010)
Full Text: HTML
Abstract
Microwave-mediated chemistry, involving the reduction of nitroarenes with molybdenum hexacarbonyl as a stoichiometric reducing agent, has been employed in the synthesis of a range of anilines. Many of these reactions exhibit high levels of chemoselectivity, tolerating unsaturation, steric hindrance and halide substituents (I, Br, Cl or F), although the latter, under certain circumstances, can be displaced in concomitant S(N)Ar/reduction processes. The reduction chemistry has been combined with palladium-catalyzed coupling and also used in the synthesis of important intermediates to kinase inhibitors or molecules with submicromolar antitrypanosomal activity. In selected cases, microwave-mediated routes have been compared with thermal (traditional oil bath) and flow reactor-mediated chemistries.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
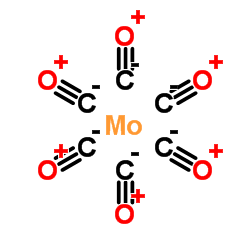 |
Carbon monooxide-molybdenum (6:1)
CAS:13939-06-5 |
C6MoO6 |
|
Determination of Ni(CO)4, Fe(CO)5, Mo(CO)6, and W(CO)6 in se...
1999-02-01 [J. Environ. Monit. 1(1) , 33-7, (1999)] |
|
In situ generation of carbon monoxide from solid molybdenum ...
2002-01-01 [J. Comb. Chem. 4(2) , 109-11, (2002)] |
|
Microwave-promoted aminocarbonylations of aryl chlorides usi...
2006-01-01 [J. Comb. Chem. 8(1) , 4-6, (2006)] |
|
Aminocarbonylations employing Mo(CO)6 and a bridged two-vial...
2012-12-21 [J. Org. Chem. 77(24) , 11393-8, (2012)] |
|
Enhanced photolysis in aerosols: evidence for important surf...
2006-10-28 [Phys. Chem. Chem. Phys. 8(40) , 4700-10, (2006)] |