| Structure | Name/CAS No. | Articles |
|---|---|---|
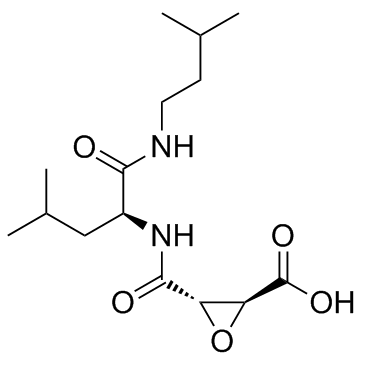 |
E-64c
CAS:76684-89-4 |
|
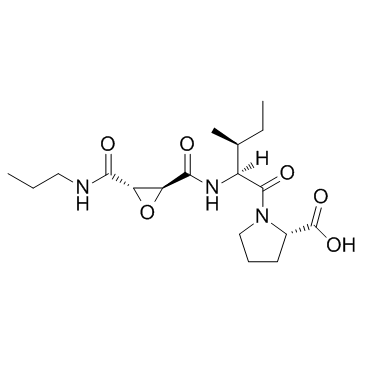 |
CA 074 TFA
CAS:134448-10-5 |