Organic Letters
2001-10-18
Chiral phosphine-free Pd-mediated asymmetric allylation of prochiral enolate with a chiral phase-transfer catalyst.
M Nakoji, T Kanayama, T Okino, Y Takemoto
Index: Org. Lett. 3(21) , 3329-31, (2001)
Full Text: HTML
Abstract
[reaction: see text]. A chiral phase-transfer catalyst has been applied to the asymmetric allylation of the tert-butyl glycinate-benzophenone Schiff base with various allylic acetates for the first time to give the allylated products in good yields and with comparable to higher enantioselectivity than for asymmetric alkylation at the same temperature (91-96% ee) without any chiral ligands for coordinating to the palladium.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
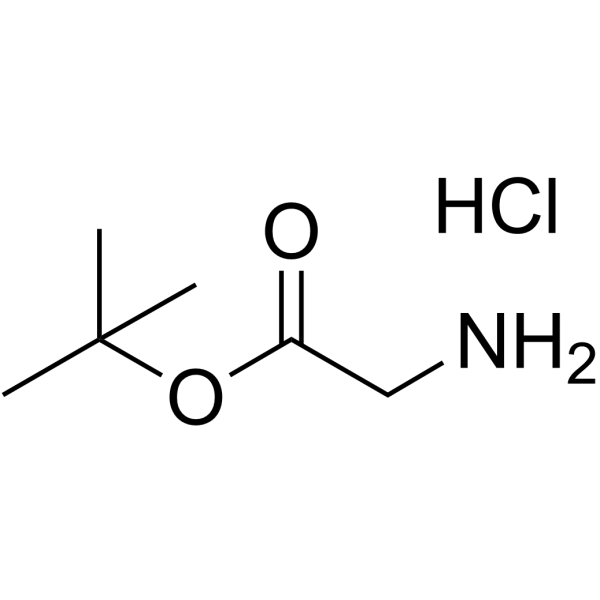 |
H-Gly-OtBu·HCl
CAS:27532-96-3 |
C6H14ClNO2 |
Related Articles:
More...
|
Inhibition of angiogenesis by antioxidant micelles.
2015-03-11 [Adv. Healthc. Mater. 4(4) , 569-75, (2015)] |
|
Synthesis and characterization of more potent analogues of h...
1998-09-29 [Biochemistry 37(39) , 13507-15, (1998)] |
|
The amplification of the anticonvulsant effect of vinyl GABA...
1984-01-01 [Arzneimittelforschung 34(6) , 687-90, (1984)] |
|
Cinchona alkaloid-based polymer-bound phase-transfer catalys...
2005-01-01 [Mol. Divers. 9(4) , 277-90, (2005)] |
|
A powerful synergistic effect for highly efficient diastereo...
2011-02-07 [Chem. Commun. (Camb.) 47(5) , 1631-3, (2011)] |