In vitro metabolism of isoxicam by horseradish peroxidase.
T F Woolf, A Black, T Chang
Index: Xenobiotica 19(12) , 1369-77, (1989)
Full Text: HTML
Abstract
1. Disposition studies in vivo in animals and man indicate that hydroxylation of the isoxazole methyl group of isoxicam is the major route of metabolism. 2. Recently, N-methylsaccharin, saccharin, and an open-ring sulphonamide have been identified as additional isoxicam metabolites. 3. Attempts to form these metabolites in vitro with hepatic microsomal incubations were unsuccessful. However, incubations of isoxicam with purified horseradish peroxidase resulted in the formation of N-methylsaccharin and the open-ring sulphonamide in good overall yield (28% in 1 h). 4. A possible mechanism for HP-catalysed conversion of isoxicam to N-methylsaccharin and open-ring sulphonamide is presented.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
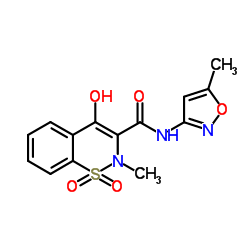 |
Isoxicam
CAS:34552-84-6 |
C14H13N3O5S |
|
Effect of some new prostaglandin synthetase inhibitors on th...
1988-04-01 [Res. Commun. Chem. Pathol. Pharmacol. 60(1) , 19-25, (1988)] |
|
[The effect of estro-progesterones on the inflammatory episo...
1989-10-01 [Rev. Rhum. Mal. Osteoartic. 56(10) , 709-11, (1989)] |
|
Controlled-release naproxen compared with isoxicam in patien...
1988-01-01 [Curr. Med. Res. Opin. 11(1) , 28-33, (1988)] |
|
In vivo metabolism of isoxicam in rats, dogs, and monkeys.
1989-01-01 [Drug Metab. Dispos. 17(6) , 662-8, (1989)] |
|
Liquid chromatography-tandem mass spectrometry method for th...
2009-04-01 [Bioanalysis 1(1) , 63-70, (2009)] |