Comparative pharmacokinetics and bioavailability of two oral formulations of thiocolchicoside, a GABA-mimetic muscle relaxant drug, in normal volunteers.
E Perucca, P Poitou, G Pifferi
Index: Eur. J. Drug Metab. Pharmacokinet. 20(4) , 301-5, (1995)
Full Text: HTML
Abstract
The comparative pharmacokinetic and bioavailability profile of two different formulations (tablets and capsules) of thiocolchicoside was investigated in 8 healthy male volunteers after administration of single oral 8 mg doses. Plasma samples were assayed by a capillary gas chromatography--mass spectrometry (GC-MS) method following enzymatic hydrolysis of thiocolchicoside to its aglycone (3-demethylthiocolchicine) and no attempt was made to account for the possible occurrence of hydrolysis in vivo. Irrespective of the formulation used, the drug was rapidly absorbed from the gastrointestinal tract, peak levels of about 17 ng/ml being detected within 1 h in most subjects. Elimination was rapid, with mean MRT values of 5-6 h. All kinetic parameters showed considerable interindividual variability but none differed significantly between the two formulations. Relative to the tablet formulation, the oral bioavailability of the capsule formulation was 1.06 +/- 0.39.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
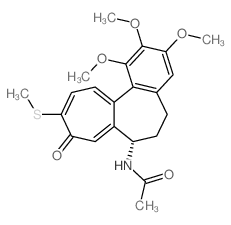 |
Thiocolchicine
CAS:2730-71-4 |
C22H25NO5S |
|
Antiproliferative activity of colchicine analogues on MDR-po...
1998-01-01 [Anticancer Drug Des. 13 , 19-33, (1998)] |
|
Antitumor agents--CLXXV. Anti-tubulin action of (+)-thiocolc...
1997-12-01 [Bioorg. Med. Chem. 5(12) , 2277-82, (1997)] |
|
Thiocolchicine dimers: a novel class of topoisomerase-I inhi...
2005-01-01 [Biochem. Pharmacol. 69(1) , 113-21, (2005)] |
|
Derivatives of thiocolchicine and its applications to CEM ce...
2010-02-01 [Bioorg. Chem. 38(1) , 1-6, (2010)] |
|
Association of thiocolchicine with tubulin.
1989-06-15 [Biochem. Biophys. Res. Commun. 161(2) , 544-50, (1989)] |