A highly enantioselective phosphabicyclooctane catalyst for the kinetic resolution of benzylic alcohols.
Edwin Vedejs, Olafs Daugulis
Index: J. Am. Chem. Soc. 125(14) , 4166-73, (2003)
Full Text: HTML
Abstract
A new class of chiral phosphines belonging to the P-aryl-2-phosphabicyclo[3.3.0]octane family (PBO) has been prepared by enantioselective synthesis starting from lactate esters and 2,2-dimethylcyclopentanone enolate 5. A selective enolate alkylation method has been developed for preparation of 9 and 10 using a chelating ester substituent in the triflate alkylating agent 11. Subsequent conversion to the PBO catalysts 2 and 39 relies on a diastereoselective cyclization from the cyclic sulfate 17 and LiPHAr to afford the more hindered endo-aryl phosphines. These phosphines function as efficient catalysts for the kinetic resolutions of aryl alkyl carbinols by benzoylation (16, 21, 22) or iso-butyroylation in the case of the less hindered aryl alkyl carbinol substrates. With o-substituted aryl alkyl carbinols, the enantioselectivities exceed 100, and s = 380 +/- 10 has been demonstrated in the case of methyl mesityl carbinol. The PBO-catalyzed acylations probably involve a P-acylphosphonium carboxylate intermediate and a tightly ion paired transition state.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
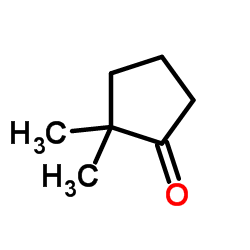 |
2,2-Dimethylcyclopentanone
CAS:4541-32-6 |
C7H12O |
|
Synthesis of novel C-2 substituted vitamin D derivatives hav...
2013-07-01 [J. Steroid Biochem. Mol. Biol. 136 , 3-8, (2013)] |
|
Synthesis of the spirosuccinimide moiety of Asperparaline A.
2000-08-01 [Biosci. Biotechnol. Biochem. 64(8) , 1758-60, (2000)] |
|
Regioselective Synthesis of 2, 2-Dimethylcyclopentanone Usin...
[Synth. Commun. 28(1) , 93-98, (1998)] |
|
Synthesis of pyrrolizidine oximes 222 and 236: Novel alkaloi...
[Tetrahedron 50(21) , 6129-36, (1994)] |
|
Synthesis of catalpalactone. Lane KJ and Pinder AR.
[J. Org. Chem. 47(16) , 3171-72, (1982)] |