Lidocaine/monoethylglycinexylidide test, galactose elimination test, and sorbitol elimination test for metabolic assessment of liver cell bioreactors.
Jörg C Gerlach, Candace Brayfield, Gero Puhl, Reiner Borneman, Christian Müller, Eva Schmelzer, Katrin Zeilinger
Index: Artif. Organs 34(6) , 462-72, (2010)
Full Text: HTML
Abstract
Various metabolic tests were compared for the performance characterization of a liver cell bioreactor as a routine function assessment of cultures in a standby for patient application in clinical studies. Everyday quality assessment (QA) is essential to ensure a continuous level of cellular functional capacity in the development of hepatic progenitor cell expansion systems providing cells for regenerative medicine research; it is also of interest to meet safety requirements in bioartificial extracorporeal liver support systems under clinical evaluation. Quality criteria for the description of bioreactor cultures were developed using primary porcine liver cells as a model. Porcine liver cells isolated by collagenase perfusion with an average of 3 x 10(9) primary cells were used in 39 bioreactors for culture periods up to 33 days. Measurements of monoethylglycinexylidide synthesis and elimination of lidocaine, galactose elimination, and sorbitol elimination proved to be useful for routine QA of primary liver cell cultures. We demonstrate two methods for dispensing test substances, bolus administration and continuous, steady-state administration. Bolus test data were grouped in Standard, Therapy, Infection/Contamination, and Cell-free control groups. Statistical analyses show significant differences among all groups for every test substance. Post hoc comparisons indicated significant differences between Standard and Cell-free groups for all elimination parameters. For continuous tests, results were categorized according to number of culture days and time-dependent changes were analyzed. Continuous administration enables a better view of culture health and the time dependency of cellular function, whereas bolus administration is more flexible. Both procedures can be used to define cell function. Assessment of cellular function and bioreactor quality can contribute significantly to the quality of experimental or clinical studies in the field of hepatic bioreactor development.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
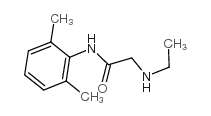 |
Monoethylglycinexylidide
CAS:7728-40-7 |
C12H18N2O |
|
Transplacental Distribution of Lidocaine and Its Metabolite ...
2015-07-01 [Reprod. Sci. 22 , 791-7, (2015)] |
|
Concentrations of dimethylaniline and other metabolites in m...
2015-01-01 [Food Addit. Contam. Part A. Chem. Anal. Control. Expo. Risk Assess. 32 , 1256-64, (2015)] |
|
MEGX test in hepatology: the long-sought ultimate quantitati...
1993-08-01 [J. Hepatol. 19(1) , 4-7, (1993)] |
|
Pharmacokinetics of lidocaine and its metabolite in peridura...
2008-12-01 [Eur. J. Clin. Pharmacol. 64(12) , 1189-96, (2008)] |
|
Effect of 4% topical lidocaine applied to the face on the se...
2010-01-01 [Aesthet. Surg. J. 30(6) , 853-8, (2010)] |