| Structure | Name/CAS No. | Articles |
|---|---|---|
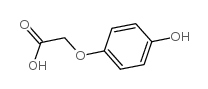 |
4-Hydroxyphenoxyacetic Acid
CAS:1878-84-8 |
Yun-Loung Lin, Jian-Lin Huang, Chung-Shieh Wu, Hung-Ge Liu, Ding-Yah Yang
Index: Bioorg. Med. Chem. Lett. 12(13) , 1709-13, (2002)
Full Text: HTML
An epoxybenzoquinone, 4-hydroxyphenoxypropionic acid, and 2-hydroxy-3-phenyl-3-butenoic acid derivatives have been designed, synthesized, and evaluated for in vitro inhibition activity against 4-hydroxyphenylpyruvate dioxygenase (4-HPPD) from pig liver by the spectrophotometric enol-borate method. The biological data demonstrated that neither epoxybenzoquinone ester nor 2-hydroxy-3-phenyl-3-butenoic acid is an inhibitor of 4-HPPD. The most potent 4-HPPD inhibitor tested was 3-hydroxy-4-phenyl-2(5H)-furanone with an IC(50) value of 0.5 microM, which may serve as a lead compound for further design of more potent 4-HPPD inhibitors.
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
4-Hydroxyphenoxyacetic Acid
CAS:1878-84-8 |
C8H8O4 |
|
Highly efficient individual dispersion of single-walled carb...
2013-02-01 [Colloids Surf. B Biointerfaces 102 , 95-101, (2013)] |
|
A synthetic heparin-mimicking polyanionic compound binds to ...
2001-01-01 [J. Cell. Biochem. 81(1) , 114-27, (2001)] |
|
Spectrophotometric assay of hydroxylated by-products in peni...
1989-06-01 [Anal. Biochem. 179(2) , 288-90, (1989)] |
|
(4-Hydroxyphenoxy) acetic acid. Byres M and Cox PJ.
[Acta Crystallogr. Sect. E Struct. Rep. Online 63(6) , o2931, (2007)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2024 ChemSrc All Rights Reserved