The behavior of organic components in copper recovery from electroless plating bath effluents using 3D electrode systems.
Gökhan Orhan, Sebahattin Gürmen, Servet Timur
Index: J. Hazard. Mater. 112(3) , 261-7, (2004)
Full Text: HTML
Abstract
An electrochemical method was applied for the recovery of copper both from the spent solutions and from the rinse waters of electroless copper plating baths, containing copper sulfate, formaldehyde, quadrol, and NaOH. Experiments were conducted in a rotating packed cell (Rollschichtzelle) to investigate the effects of current density, electrolyte composition, temperature, and pH on the copper recovery. All the copper (final CCu=0.1 ppm) was recovered from the waste and rinse waters of chemical copper plating plants with 70% current efficiency by the electrochemical treatment in a rotating packed cell at 130 A/m2 current density, room temperature, with 5mm diameter cathode granules, with the presence of formaldehyde, and with a specific energy consumption of 3.2-3.5 kW h/kg Cu. On the other hand, final copper concentrations of 5 ppm were reached with 62% current efficiency and 5.5-5.8 kW h/kg Cu specific energy consumption, with electrolytes containing no formaldehyde.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
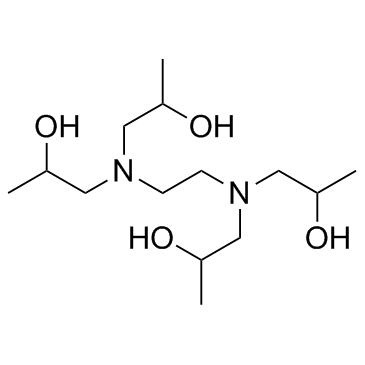 |
Edetol
CAS:102-60-3 |
C14H32N2O4 |
|
Autoclavable highly cross-linked polyurethane networks in op...
1993-11-01 [Biomaterials 14(14) , 1089-97, (1993)] |
|
Sorption of Cu(II) complexes with ligands tartrate, glycine ...
2009-11-15 [J. Hazard. Mater. 171(1-3) , 133-9, (2009)] |
|
In vitro stimulation of macrophages by quadrol [N,N,N',N'-te...
1985-01-01 [J. Immunopharmacol. 7(3) , 303-12, (1985)] |
|
Decontamination of solutions containing Cu(II) and ligands t...
2010-03-15 [J. Hazard. Mater. 175(1-3) , 452-9, (2010)] |
|
Evaluation of the efficacy of a topical cosmetic slimming pr...
2011-12-01 [Int. J. Cosmet. Sci. 33(6) , 519-26, (2011)] |