| Structure | Name/CAS No. | Articles |
|---|---|---|
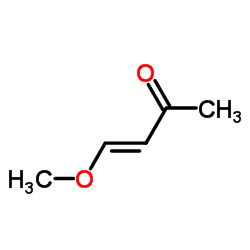 |
(3E)-4-Methoxy-3-buten-2-one
CAS:51731-17-0 |
| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
(3E)-4-Methoxy-3-buten-2-one
CAS:51731-17-0 |