Inhibition of HIV-associated reverse transcriptase by sugar-modified derivatives of thymidine 5'-triphosphate in comparison to cellular DNA polymerases alpha and beta.
E Matthes, C Lehmann, D Scholz, M von Janta-Lipinski, K Gaertner, H A Rosenthal, P Langen
Index: Biochem. Biophys. Res. Commun. 148(1) , 78-85, (1987)
Full Text: HTML
Abstract
The sugar-modified dTTP analogues 2',3'-didehydro-2',3'-dideoxy-thymidine 5'-triphosphate (ddeTTP), 2',3'-dideoxythymidine 5'-triphosphate (ddTTP), 3'-fluorothymidine 5'-triphosphate (FdTTP), and 3'-azidothymidine 5'-triphosphate (N3dTTP) are demonstrated to be very effective and selective inhibitors of the HIV-associated reverse transcriptase (HIV-RT). This conclusion is based on a comparison of the ID50 values of the compounds for the HIV-RT (ranging from 0.03 microM for ddeTTP to 0.1 microM for ddTTP) and the cellular DNA polymerase alpha (greater than 200 microM). DNA polymerase beta is partially affected by N3dTTP (ID50 = 31 microM) and by the other analogues (ID50 = 1-2.2 microM). FdTTP has proved as effective as N3dTTP (ID50 = 0.05 microM) in suppressing the HIV-RT activity. Kinetic analysis revealed for both dTTP analogues a competitive type of inhibition and the same K1 values (about 0.05 microM).
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
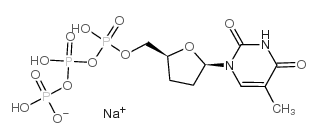 |
3'-deoxythymidine-5'-o-triphosphate/2',3'-dideoxythymidine-5'-o-triphosphate sodium salt
CAS:128524-26-5 |
C10H16N2NaO13P3 |
|
Nicked-site substrates for a serine recombinase reveal enzym...
2015-07-13 [Nucleic Acids Res. 43 , 6134-43, (2015)] |
|
Development of bis-locked nucleic acid (bisLNA) oligonucleot...
2013-03-01 [Nucleic Acids Res. 41 , 3257-73, (2013)] |
|
A combined HM-PCR/SNuPE method for high sensitive detection ...
2010-01-01 [Epigenetics Chromatin 3 , 12, (2010)] |
|
Inhibition of the activity of DNA polymerase alpha by 2',3'-...
1979-06-27 [Biochem. Biophys. Res. Commun. 88(4) , 1255-62, (1979)] |
|
Polynucleotide phosphorylase exonuclease and polymerase acti...
2011-11-01 [Nucleic Acids Res. 39(21) , 9250-61, (2011)] |