Highly selective rhodium-catalyzed conjugate addition reactions of 4-oxobutenamides.
Jamie L Zigterman, Jacqueline C S Woo, Shawn D Walker, Jason S Tedrow, Christopher J Borths, Emilio E Bunel, Margaret M Faul
Index: J. Org. Chem. 72 , 8870, (2007)
Full Text: HTML
Abstract
A variety of 4-oxobutenamides 1 were subjected to rhodium-catalyzed conjugate addition with arylboronic acids providing high regio- and enantioselectivity (97:3 to >99:1, >96% ee) and moderate to excellent yields (54-99%). The key to high selectivity is the use of sterically demanding P-chiral diphosphines, such as Tangphos or Duanphos. The product oxobutanamides 2 may be converted to alternate targets by selective derivatization of either the amide or ketone functional group. A stereochemical model predicting the absolute sense of induction was developed based on single-crystal X-ray structures of product and precatalyst.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
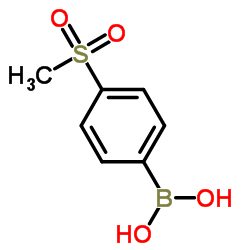 |
4-(methylsulfonyl)phenylboronic acid
CAS:149104-88-1 |
C7H9BO4S |
|
3,5-Diaryl-2-aminopyridines as a novel class of orally activ...
2012-04-12 [J. Med. Chem. 7th ed., 55 , 3479-3487, (2012)] |
|
Copper-catalyzed oxidative trifluoromethylthiolation of aryl...
2012-03-05 [Angew. Chem. Int. Ed. Engl. 10th ed., 51 , 2492-2495, (2012)] |
|
Synthesis of unsymmetrical 3,4-diaryl-3-pyrrolin-2-ones util...
2011-10-21 [J. Org. Chem. 76 , 8203-8214, (2011)] |
|
Synthesis and biological evaluation of new 3-(6-hydroxyindol...
2011-11-01 [Eur. J. Med. Chem. 11th ed., 46 , 5416-5434, (2011)] |
|
The design, synthesis and biological evaluations of C-6 or C...
2012-01-01 [Bioorg. Med. Chem. 1st ed., 20 , 467-479, (2012)] |