Ion-pair formation of Bi(III)-iodide with some nitrogenous drugs and its application to pharmaceutical preparations.
F M Abdel-Gawad
Index: J. Pharm. Biomed. Anal. 16(5) , 793-9, (1998)
Full Text: HTML
Abstract
A systematic spectrophotometric study on the ion-pair formation of Bi(III)-iodide with amineptine hydrochloride, piribedil and trimebutine maleate is carried out. The optimal experimental conditions pH, concentration of Bi(III) nitrate, potassium iodide; and the nature and amount of organic solvent have been studied. The ion pairs are soluble in 1,2-dichloroethane and the optimum pH range is 2.0-2.8. By application of the methods of Sommer and Job involving non-equimolar solutions, the conditional stability constant (log K') of the Bi(III) piridedil ion pair (1:1) at the optimum pH of 2.4 and an ionic strength (mu) 0.1 M, was found to be 5.436. The validity of Beer's law has been tested in the concentration range 5-50 microg ml(-1) in the organic layer, the relative standard deviation is less than 1%. The method is applied to the determination of these drugs in tablets without interference.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
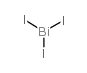 |
Bismuthine, triiodo
CAS:7787-64-6 |
BiI3 |
|
Spray pyrolysis deposition and photoelectrochemical properti...
2012-09-25 [ACS Nano 6(9) , 7712-22, (2012)] |
|
Enhanced adsorption and photocatalytic activity of BiOI-MWCN...
2012-08-30 [J. Hazard. Mater. 229-230 , 72-82, (2012)] |
|
Iodobismuthates with N-alkyl- or N,N'-dialkyl-4,4'-bipyridin...
2011-04-07 [Phys. Chem. Chem. Phys. 13(13) , 5659-67, (2011)] |
|
Reversible dynamic isomerism change in the solid state, from...
2008-11-30 [Chem. Commun. (Camb.) (44) , 5743-5, (2008)] |
|
Bismuth iodide impregnation of the peripheral nerve fibres.
1985-01-01 [Z. Mikrosk. Anat. Forsch. 99(5) , 804-12, (1985)] |