Journal of Organic Chemistry
2012-07-20
Aminoindolines versus quinolines: mechanistic insights into the reaction between 2-aminobenzaldehydes and terminal alkynes in the presence of metals and secondary amines.
Nitin T Patil, A Nijamudheen, Ayan Datta
Index: J. Org. Chem. 77(14) , 6179-85, (2012)
Full Text: HTML
Abstract
DFT computational studies in the cyclization of aminoalkyne (see structure), which is generated in situ by 2-aminobenzaldehydes and terminal alkynes in the presence of metals and secondary amines, has been investigated. The study revealed that the mode of cyclization (exo vs endo) depends on the protecting group on nitrogen, the oxidation state of copper, and substitution on alkyne.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
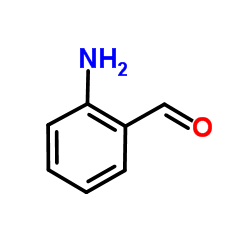 |
Formylaniline
CAS:529-23-7 |
C7H7NO |
Related Articles:
More...
|
Chondrogenesis of human bone marrow mesenchymal stromal cell...
2015-05-01 [Mater. Sci. Eng. C. Mater. Biol. Appl. 50 , 160-72, (2015)] |
|
B-ring-aryl substituted luotonin A analogues with a new bind...
2014-01-01 [PLoS ONE 9(5) , e95998, (2014)] |
|
The consequences of the isotope effect on proline dehydrogen...
1992-06-01 [Anal. Biochem. 203(2) , 191-200, (1992)] |
|
Co-regulation of mitochondrial respiration by proline dehydr...
2016-03-01 [Amino Acids 48 , 859-72, (2016)] |
|
Functional specialization in proline biosynthesis of melanom...
2012-01-01 [PLoS ONE 7 , e45190, (2012)] |