Discovery of a novel selective PPARgamma modulator from (-)-Cercosporamide derivatives.
Akihiro Furukawa, Tsuyoshi Arita, Susumu Satoh, Kenji Wakabayashi, Shinko Hayashi, Yumi Matsui, Kazushi Araki, Masanori Kuroha, Jun Ohsumi
Index: Bioorg. Med. Chem. Lett. 20(7) , 2095-8, (2010)
Full Text: HTML
Abstract
In an investigation of (-)-Cercosporamide derivatives with a plasma glucose-lowering effect, we found that N-benzylcarboxamide derivative 4 was a partial agonist of PPARgamma. A SAR study of the substituents on carboxamide nitrogen afforded the N-(1-naphthyl)methylcarboxamide derivative 23 as the most potent selective PPARgamma modulator. An X-ray crystallography study revealed that compound 23 bounded to the PPARgamma ligand binding domain in a unique way without any interaction with helix12. Compound 23 displayed a potent plasma glucose-lowering effect in db/db mice without the undesirable increase in body fluid and heart weight that is typically observed when PPARgamma full agonists are administrated.2010 Elsevier Ltd. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
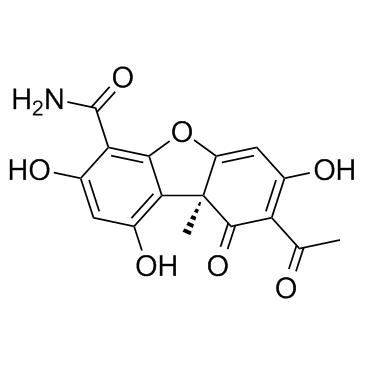 |
Cercosporamide
CAS:131436-22-1 |
C16H13NO7 |
|
Bioactive metabolites from Phoma species, an endophytic fung...
2012-02-01 [Appl. Microbiol. Biotechnol. 93(3) , 1231-9, (2012)] |
|
Inhibition of Mnk kinase activity by cercosporamide and supp...
2013-05-02 [Blood 121(18) , 3675-81, (2013)] |
|
Cloning and characterization of KNR4, a yeast gene involved ...
1994-02-01 [Mol. Cell. Biol. 14(2) , 1017-25, (1994)] |
|
Fermentative production of self-toxic fungal secondary metab...
2010-04-01 [J. Ind. Microbiol. Biotechnol. 37(4) , 335-40, (2010)] |
|
Synthesis and biological evaluation of novel (-)-Cercosporam...
2012-08-01 [Eur. J. Med. Chem. 54 , 522-33, (2012)] |