1,4-Dihydropyridines bearing a pharmacophoric fragment of lidoflazine.
A Chiarini, A Rampa, R Budriesi, A Bisi, G Fabbri, P Valenti
Index: Bioorg. Med. Chem. 4(10) , 1629-35, (1996)
Full Text: HTML
Abstract
A series of 1,4-dihydropyridines bearing a pharmacophoric fragment of lidoflazine was synthesized. The compounds were evaluated for inotropic, chronotropic, and calcium antagonist activities. All compounds behave as inotropic and chronotropic agents, except for compounds 4b, 5a, and 5b, which exhibit a rather weak calcium antagonism in vascular smooth muscle (like aorta). Compound 5b is about twofold more potent in decreasing both chronotropy and inotropy, while compound 5c is about fivefold more potent in decreasing inotropy than nifedipine. Moreover, compound 5b is the most potent calcium antagonist derivative of the series.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
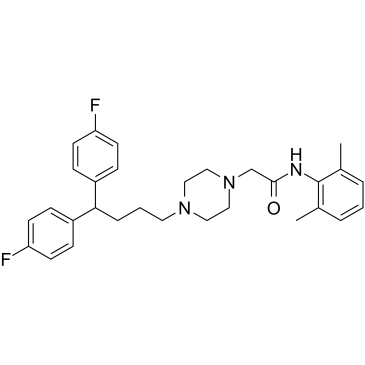 |
Lidoflazine
CAS:3416-26-0 |
C30H35F2N3O |
|
Protective effects of the lazaroid U74500A and lidoflazine o...
1993-01-01 [Transpl. Int. 6(5) , 281-4, (1993)] |
|
Simpson's paradox and clinical trials: what you find is not ...
1992-12-01 [Ann. Emerg. Med. 21(12) , 1480-2, (1992)] |
|
Effects of lidoflazine on left ventricular function in patie...
1997-02-01 [J. Cardiothorac. Vasc. Anesth. 11(1) , 42-8, (1997)] |
|
The pathophysiology of angina pectoris and the effect of lid...
1982-01-01 [Circulation 65(1 Pt 2) , I27-32, (1982)] |
|
Lidoflazine and myocardial protection.
1995-05-01 [J. Thorac. Cardiovasc. Surg. 109(5) , 1013-4; discussion 1015, (1995)] |