Mature experimental constructed wetlands treating urban water receiving high metal loads.
Miklas Scholz, Patrick Höhn, Rowland Minall
Index: Biotechnol. Prog. 18(6) , 1257-64, (2002)
Full Text: HTML
Abstract
The aim was to assess over 2 years the treatment efficiencies of vertical-flow wetland filters containing macrophytes and granular media of different adsorption capacities. Different concentrations of lead and copper sulfate (constant for 1 year each) were added to urban beck inflow water in order to simulate pretreated (pH adjustment assumed) mine wastewater. After 1 year of operation, the inflow concentrations for lead and copper were increased from 1.30 to 2.98 and from 0.98 to 1.93 mg/L, respectively. However, the metal mass load rates (mg/m(2)/d) were increased by a factor of approximately 4.9 for lead and 4.3 for copper. No breakthrough of metals was recorded. Lead and copper accumulated in the biomass of the litter zone and rhizomes of the macrophytes. Furthermore, microbiological activity decreased during the second year of operation. Bioindicators such as ciliated protozoa and zooplankton decreased sharply in numbers but diatoms increased. In conclusion, the use of macrophytes and adsorption media did not significantly enhance the filtration of lead and copper. Particulate lead is removed by filtration processes including straining. Furthermore, some expensive and time-consuming water quality variables can be predicted with less expensive ones such as temperature in order to reduce sampling costs.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
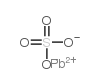 |
Lead sulfate
CAS:7446-14-2 |
O4PbS |
|
Chemical transformations of lead compounds under humid condi...
2013-02-01 [Environ. Geochem. Health 35(1) , 153-9, (2013)] |
|
Phosphate-induced lead immobilization from different lead mi...
2008-03-01 [Environ. Pollut. 152(1) , 184-92, (2008)] |
|
The effects of lead sulfate on new sealed lead acid batterie...
2000-04-01 [J. Emerg. Med. 18(3) , 305-9, (2000)] |
|
Low temperature catalytic conversion of methane to methanol ...
2005-05-07 [Chem. Commun. (Camb.) (17) , 2238-40, (2005)] |
|
Heterogeneous chemistry between PbSO4 and calcite microparti...
2006-08-01 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 64(5) , 1095-101, (2006)] |