Mutagenicity of bay-region amino-substituted cyclopenta[a]phenanthrenes and 2- and 5-aminochrysene.
F S Catterall, M M Coombs, C Ioannides, J J Sepiol, J Wilson
Index: Mutat. Res. 492(1-2) , 7-11, (2001)
Full Text: HTML
Abstract
The relative mutagenic potentials of 11-amino-16,17-dihydro-15H-cyclopenta[a]phenanthrene, its 17-keto derivative, and 2- and 5-aminochrysene have been compared in Salmonella typhimurium TA98 and TA100 in the presence of a postmitochondrial liver preparation from Aroclor 1254 induced rats. The 11-amino hydrocarbon is a very weak mutagen (0.27 revertants/nmol), whereas the 11-amino-17-ketone is much more active (129 revertants/nmol). 2-Aminochrysene is the most mutagenic arylamine ( approximately 500 revertants/nmol) among these compounds, but its 5-amino isomer is much less active (0.9 revertants/nmol). Possible reasons for these marked differences are suggested. Use of TA98 with over-expressing O-acetyltransferase (YG 1024) and deficient in this enzyme (TA98/l,8-DNP(6)) with the 11-amino-17-ketone and with 5-aminochrysene clearly indicates the importance of this enzyme in their bioactivation, implying oxidation of the amino group to the hydroxylamine in both these compounds.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
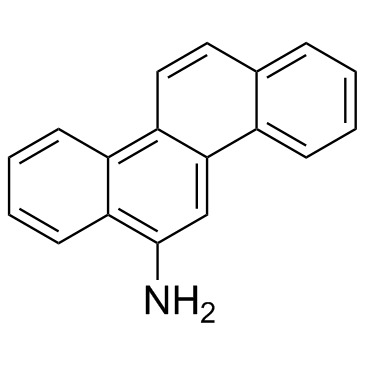 |
6-Aminochrysene
CAS:2642-98-0 |
C18H13N |
|
Role of human N-acetyltransferases, NAT1 or NAT2, in genotox...
1999-06-01 [Carcinogenesis 20(6) , 1079-83, (1999)] |
|
Quantitative microspectrofluorimetry study of the "blocking ...
1984-02-01 [Toxicology 29(4) , 345-56, (1984)] |
|
Determination of polycyclic aromatic amines in skin by liqui...
[J. Chromatogr. A. 354 , 442-8, (1986)] |
|
Activation of trans-1,2-dihydro-1,2-dihydroxy-6-aminochrysen...
1994-03-01 [Carcinogenesis 15(3) , 465-70, (1994)] |
|
Induction of mutations at the hypoxanthine phosphoribosyl tr...
1994-10-01 [Mutat. Res. 310(1) , 45-54, (1994)] |