Organic Letters
2009-08-20
Access to a welwitindolinone core using sequential cycloadditions.
Barry M Trost, Patrick J McDougall
Index: Org. Lett. 11(16) , 3782-5, (2009)
Full Text: HTML
Abstract
A concise approach to the core skeleton of the welwitindolinone alkaloids was developed on the basis of sequential cycloaddition reactions. First, a palladium catalyzed enantioselective [6 + 3] trimethylenemethane cycloaddition onto a tropone nucleus was used to generate the requisite bicyclo[4.3.1]decadiene. Subsequent modifications to the cycloadduct allowed for an intramolecular [4 + 2] cycloaddition to generate the oxindole and complete the core of the natural product family.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
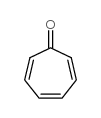 |
TROPONE
CAS:539-80-0 |
C7H6O |
Related Articles:
More...
|
Benzo[cd]azulene skeleton: azulene, heptafulvene, and tropon...
2009-12-03 [Org. Lett. 11(23) , 5363-5, (2009)] |
|
Tropylium tetrafluoroborate, a novel substrate for aldehyde ...
1986-10-30 [Biochem. Biophys. Res. Commun. 140(2) , 609-15, (1986)] |
|
Potential of Piperazinylalkylester Prodrugs of 6-Methoxy-2-N...
2015-06-01 [Z. Naturforsch., C, J. Biosci. 59(3-4) , 184-6, (2004)] |
|
Pernambucone, a new tropone derivative from Croton argyroglo...
2009-05-01 [Pharmazie 64(5) , 350-1, (2009)] |
|
Metal-catalyzed [6 + 3] cycloaddition of tropone with azomet...
2014-02-12 [J. Am. Chem. Soc. 136(6) , 2625-9, (2014)] |