| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Cis-2-Amino-1-Cyclohexanecarboxylic Acid
CAS:45743-49-5 |
|
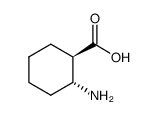 |
trans-2-amino-1-cyclohexanecarboxylic acid
CAS:5691-19-0 |