Properties of the Escherichia coli rhodanese-like protein SseA: contribution of the active-site residue Ser240 to sulfur donor recognition.
R Colnaghi, G Cassinelli, M Drummond, F Forlani, S Pagani
Index: FEBS Lett. 500(3) , 153-6, (2001)
Full Text: HTML
Abstract
The product of Escherichia coli sseA gene (SseA) was the subject of the present investigation aimed to provide a tool for functional classification of the bacterial proteins of the rhodanese family. E. coli SseA contains the motif CGSGVTA around the catalytic cysteine (Cys238). In eukaryotic sulfurtransferases this motif discriminates for 3-mercaptopyruvate:cyanide sulfurtransferase over thiosulfate:cyanide sulfurtransferases (rhodanese). The biochemical characterization of E. coli SseA allowed the identification of the first prokaryotic protein with a preference for 3-mercaptopyruvate as donor substrate. Replacement of Ser240 with Ala showed that the presence of a hydrophobic residue did not affect the binding of 3-mercaptopyruvate, but strongly prevented thiosulfate binding. On the contrary, substitution of Ser240 with an ionizable residue (Lys) increased the affinity for thiosulfate.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
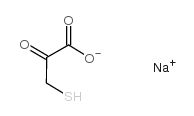 |
sodium mercaptopyruvate
CAS:10255-67-1 |
C3H3NaO3S |
|
Structure and kinetic analysis of H2S production by human me...
2013-07-05 [J. Biol. Chem. 288(27) , 20002-13, (2013)] |
|
Identification of putative sulfurtransferase genes in the ex...
2005-04-01 [OMICS 9(1) , 13-29, (2005)] |
|
Novel, orally effective cyanide antidotes.
2007-12-27 [J. Med. Chem. 50(26) , 6462-4, (2007)] |
|
Alternative sulphur donors for detoxification of cyanide in ...
1991-01-01 [Comp. Biochem. Physiol. C, Comp. Pharmacol. Toxicol. 99(3) , 309-15, (1991)] |
|
Electron paramagnetic resonance evidence for a cysteine-base...
1995-05-02 [Biochemistry 34(17) , 5712-7, (1995)] |