Renal brush border enzyme-cleavable linkages for low renal radioactivity levels of radiolabeled antibody fragments.
Hiromichi Akizawa, Mitsuo Imajima, Hirofumi Hanaoka, Tomoya Uehara, Satoshi Satake, Yasushi Arano
Index: Bioconjug. Chem. 24(2) , 291-9, (2013)
Full Text: HTML
Abstract
We previously demonstrated that Fab fragments labeled with 3'-[(131)I]iodohippuryl N(ε)-maleoyl-l-lysine ([(131)I]HML) showed low renal radioactivity from early postinjection time, due to a liberation of m-[(131)I]iodohippuric acid by the action of renal brush border enzymes. Since there are lots of enzymes on renal brush border membrane, peptide linkages other than the glycyl-l-lysine were evaluated as the cleavable linkages to explore the chemical design. In this study, we evaluated four peptide linkages with a general formula of m-iodobenzoyl-glycyl-X (X: l-tyosine O-methyl, l-asparagine, l-glutamine, and N(ε)-Boc-l-lysine). In vitro studies using renal brush border membrane vesicles (BBMVs) demonstrated that 3'-[(125)I]iodohippuryl O-methyl-l-tyrosine (2c) liberated the highest amount of m-[(125)I]iodohippuric acid among the four substrates and the change in the linkage structure altered enzyme species responsible for the hydrolysis reaction. To further assess the applicability of the linkage, a radioiodination reagent containing a glycyl-tyrosine linkage, 3'-[(125)I]iodohippuryl O-((2-maleimidoethyl)carbamoyl)methyl-l-tyrosine (HMT, 12c), was designed, synthesized, and subsequently conjugated to an Fab fragment. [(125)I]HMT-Fab exhibited renal radioactivity levels similar to and significantly lower than [(125)I]HML-Fab and directly radioiodinated Fab, while the blood clearance rates of the three were similar. The analyses of urine for 24 h postinjection of [(125)I]HMT-Fab showed that m-[(125)I]iodohippuric acid was excreted as the major radiometabolite. The findings indicated that glycyl-tyrosine linkage is also available to reduce renal radioactivity levels of radioiodinated Fab fragments, due to liberation of m-iodohippuric acid by the action of enzymes present on renal brush border membrane. These findings suggest that an appropriate selection of peptide linkages would allow the liberation of a designed radiolabeled compound from covalently conjugated polypeptides to prepare radiolabeled polypeptides of low renal radioactivity levels. For the selection of the most appropriate peptide linkage, the in vitro system using BBMVs would be useful to narrow the candidates to just a few.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
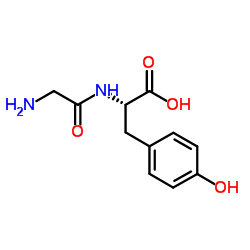 |
H-Gly-Tyr-OH
CAS:658-79-7 |
C11H14N2O4 | |
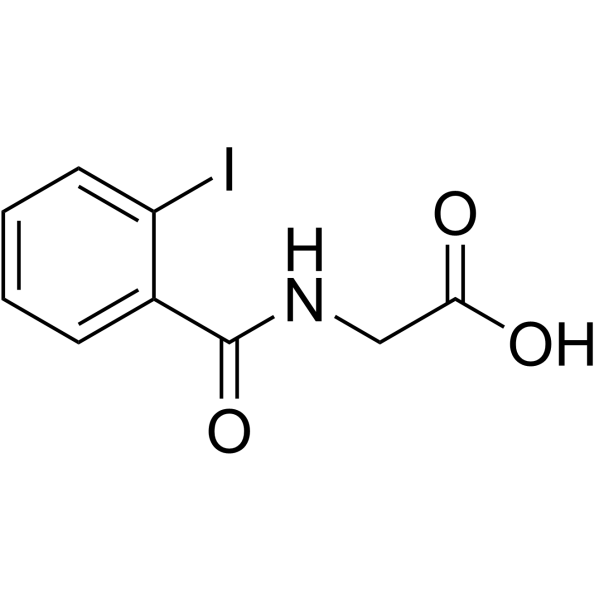 |
2'-iodohippuric acid
CAS:147-58-0 |
C9H8INO3 |
|
Analysis of peptides and protein digests by reversed phase h...
2015-05-04 [Anal. Chim. Acta 872 , 84-94, (2015)] |
|
[Studies on iodinated compounds. X. Isolation and purificati...
1999-09-01 [Yakugaku Zasshi 119(9) , 681-7, (1999)] |
|
Conformational energies of substrates and inhibitors for car...
1995-04-01 [J. Biomol. Struct. Dyn. 12(5) , 1033-40, (1995)] |
|
Human PEPT1 pharmacophore distinguishes between dipeptide tr...
2006-06-15 [J. Med. Chem. 49 , 3636-44, (2006)] |
|
Parenteral peptide supplements after major surgery.
1989-05-13 [Lancet 1(8646) , 1085-6, (1989)] |