Combination of trimethoprim with sulfonamides other than sulfamethoxazole.
L S Bernstein
Index: Rev. Infect. Dis. 4(2) , 411-8, (1982)
Full Text: HTML
Abstract
Early in the development of trimethoprim (TMP), the response to the drug was less than enthusiastic. Preliminary clinical trials were performed with sulfonamides that were unsuitable partners. On the basis of bacteriologic and pharmacokinetic evidence, sulfamethoxazole (SMZ) was chosen for the fixed-ratio (5:1) combination with TMP. Possible criteria on which to base the choice of a sulfonamide partner for TMP have been delineated. The theoretical considerations may be questioned in view of limited clinical experience with some combinations and lack of controlled comparisons in well-designed clinical trials with others. Objective proof of the clinical superiority of newer TMP-sulfonamide combinations over TMP-SMZ is required.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
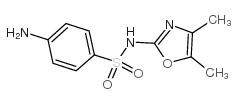 |
sulfamoxol
CAS:729-99-7 |
C11H13N3O3S |
|
Adverse reactions to sulphonamide and sulphonamide-trimethop...
1996-03-01 [Adverse Drug React. Toxicol. Rev. 15(1) , 9-50, (1996)] |
|
Use of p-benzoquinone for the spectrophotometric determinati...
1991-01-01 [J. Pharm. Biomed. Anal. 9(7) , 531-8, (1991)] |
|
Comparative pharmacokinetics of co-trifamole and co-trimoxaz...
1982-09-01 [Br. J. Clin. Pharmacol. 14(3) , 437-43, (1982)] |
|
[Supristol suspension in pediatrics].
1980-12-01 [Lille Med. 25(10) , 611-2, (1980)] |
|
Ondansetron attenuates co-morbid depression and anxiety asso...
2015-09-01 [J. Endocrinol. 91(2) , 299-303, (1981)] |