[Modification of surface residues of lysine in immunoglobulin G using the spin marker 2,2,5,5-tetramethyl-3-maleimidopyrrolidine-1-oxyl].
V A Lapuk, E Iu Varlamova, A P Mozoleva, N V Anisimov, V P Timofeev
Index: Biofizika 44(5) , 806-10, (1999)
Full Text: HTML
Abstract
Exposed lysine residues of human IgG were modified by a spin-label, 2,2,5,5-tetramethyl-3-male-imidopyrrolidine-1-oxyl at pH 9.2. Under these conditions, the degree of modification was about 10 lysine residues per protein molecule. The ESR spectrum of the spin-labeled immunoglobulin was much more mobile than that of spin-labeled immunoglobulin with the modification degree of about 1 residue that was obtained at pH 7.0. Thus, the sharp increase in the modification degree due to the increase in pH by two units leads to a marked loosening of the tertiary structure of the protein in solution, which is just indicated by the mobile ESR spectrum. Lithium chloride added to the solution of spin-labeled immunoglobulin induces a similar "immobilization" of its ESR spectra as sucrose.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
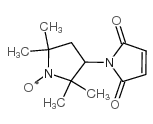 |
3-Maleimido-PROXYL
CAS:5389-27-5 |
C12H17N2O3 |
|
EPR and NMR spectroscopies provide input on the coordination...
2015-06-01 [J. Biol. Inorg. Chem. 20 , 719-27, (2015)] |
|
A comparison between interactions of triglyceride oil and mi...
2015-08-01 [Int. J. Cosmet. Sci. 37 , 371-8, (2015)] |
|
Interaction of transmembrane helices in ATP synthase subunit...
2008-02-01 [Biochim. Biophys. Acta 1777(2) , 227-37, (2008)] |
|
The myosin catalytic domain does not rotate during the worki...
1995-09-01 [Biophys. J. 69(3) , 994-9, (1995)] |
|
Membrane assembly of the 16-kDa proteolipid channel from Nep...
1999-10-26 [Biochemistry 38(43) , 14311-9, (1999)] |