Differences in lercanidipine systemic exposure when administered according to labelling: in fasting state and 15 minutes before food intake.
Covadonga Álvarez, Estrella Gómez, Marta Simón, Carlos Govantes, Pedro Guerra, Jesús Frías, Alfredo García-Arieta
Index: Eur. J. Clin. Pharmacol. 68(7) , 1043-7, (2012)
Full Text: HTML
Abstract
The aim of this study was to compare the systemic exposure of lercanidipine (Zanidip) after oral administration in the fasted state and 15 min before food intake (meals) to investigate if the recommendations in the Summary of Product Characteristics (SPC) with respect to the intake of meals are adequate.The results of three pilot bioequivalence studies performed to develop a lercanidipine generic product, where Zanidip was administered consistently as reference product in the fasted state or 15 min before a standard breakfast, were compared to estimate the drug–food interaction and the similarity of the methods of administration defined in the SPC.The ingestion of a standard (non-high-fat, non-high-calorie) meal 15 min after drug intake increased the area under the concentration–time curve (AUC(0-t)) of S-lercanidipine by 1.78-fold [90% confidence interval (CI) 1.48–2.15, P<0.0001] and the maximum concentration (Cmax) of Slercanidipine by 1.82-fold (90% CI 1.46–2.28, P<0.0001). These values are close to the twofold increase that has been described when Zanidip was taken immediately after a carbohydrate-rich meal. Higher levels would be expected with a high-fat, high-calorie meal.As intake with a carbohydrate-rich meal is not recommended in the SPC of Zanidip because a twofold difference was considered to be clinically relevant, the intake of lercanidipine only 15 min before food intake does not seem to be consistent with this recommendation. The Marketing Authorisation Holder should clarify the dosing instructions in relation to meals and identify a sufficient time-lapse to ensure an exposure similar to that obtained in phase III clinical efficacy studies.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
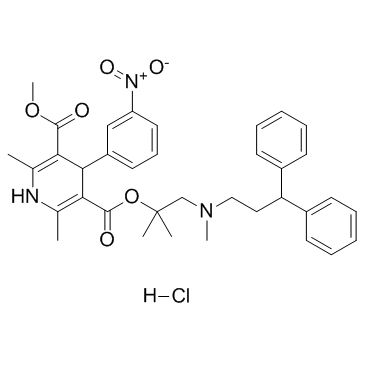 |
Lercanidipine hydrochloride
CAS:132866-11-6 |
C36H42ClN3O6 |
|
Rationale for the use of a fixed-dose combination in the man...
2010-01-01 [Clin. Drug Investig. 30(12) , 843-54, (2010)] |
|
Lercanidipine rescues hippocampus pyramidal neurons from mil...
2011-05-01 [Cell. Mol. Neurobiol. 31(4) , 561-7, (2011)] |
|
Incidence and clinical course of lercanidipine-associated cl...
2010-09-01 [Clin. Nephrol. 74(3) , 217-22, (2010)] |
|
Polymorphism of the methylenetetrahydrofolate reductase gene...
2012-06-01 [Gene 500(2) , 207-10, (2012)] |
|
Efficacy and tolerability of the fixed lercanidipine-enalapr...
2010-01-01 [Arzneimittelforschung 60(3) , 124-30, (2010)] |