| Structure | Name/CAS No. | Articles |
|---|---|---|
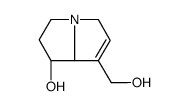 |
Retronecine
CAS:480-85-3 |
|
![(1S)-1-[2,4,6-tri(propan-2-yl)phenyl]ethanol Structure](https://image.chemsrc.com/caspic/177/102225-88-7.png) |
(1S)-1-[2,4,6-tri(propan-2-yl)phenyl]ethanol
CAS:102225-88-7 |
|
![METHYL 8-CHLORO-6-(TRIFLUOROMETHYL)IMIDAZO[1,2-A]PYRIDINE-7-CARBOXYLATE Structure](https://image.chemsrc.com/caspic/208/181531-14-6.png) |
METHYL 8-CHLORO-6-(TRIFLUOROMETHYL)IMIDAZO[1,2-A]PYRIDINE-7-CARBOXYLATE
CAS:181531-14-6 |