Molecular Diversity
2012-05-01
Synthesis of 5-alkylated barbituric acids and 3-alkylated indoles via microwave-assisted three-component reactions in solvent-free conditions using Hantzsch 1,4-dihydropyridines as reducing agents.
Biswajita Baruah, P Seetham Naidu, Pallabi Borah, Pulak J Bhuyan
Index: Mol. Divers. 16(2) , 291-8, (2012)
Full Text: HTML
Abstract
Reaction of barbituric acids with aldehydes and dihydropyridines in one pot under microwave (MW) irradiation in the absence of solvent, affords 55–82% of the 5-benzylated barbituric acids. Use of alkyl nitriles or barbituric acids with indole-3-aldehyde and dihydropyridine (DHP) afforded 3-alkylated indoles in 57–76 % yield. In each case DHPs are converted to pyridines.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
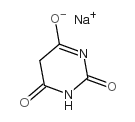 |
sodium barbiturate
CAS:4390-16-3 |
C4H3N2NaO3 |
Related Articles:
More...
|
Identification and characterization of Clostridium perfringe...
2015-04-01 [Infect. Immun. 83(4) , 1477-86, (2015)] |
|
Characterization of two long-chain fatty acid CoA ligases in...
2012-12-20 [Microbiol. Res. 167(10) , 602-7, (2012)] |
|
Synergistic effects of Clostridium perfringens enterotoxin a...
2014-07-01 [Infect. Immun. 82(7) , 2958-70, (2014)] |
|
Complementary hydrogen bonding of a carboxylato-barbiturate ...
2011-12-01 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 83(1) , 532-9, (2011)] |
|
Barbiturate ingestion in three adult captive tigers (Panther...
2011-12-01 [J. S. Afr. Vet. Assoc. 82(4) , 244-9, (2011)] |