Purification of rat kidney carboxylesterase and its comparison with other tissue esterases.
T Tsujita, T Miyada, H Okuda
Index: J. Biochem. 103(2) , 327-31, (1988)
Full Text: HTML
Abstract
Carboxylesterase was purified from rat kidney in an electrophoretically homogeneous form by acetone precipitation, followed by successive chromatographies on DEAE-cellulose and hydroxyapatite and then isoelectric focusing. The purified enzyme catalyzed the hydrolyses of monoacylglycerols and short-chain triacylglycerols, such as tributyrin, but not the hydrolysis of long-chain triacylglycerol. Its optimum pH with methyl butyrate as a substrate was 8.0. The relation of its activity to the methyl butyrate concentration differed from those for pancreatic lipase and liver esterase, and also from those for lipolytic enzymes from various other tissues. The relations of methyl butyrate-hydrolyzing activity with methyl butyrate concentration were compared among various carboxylester hydrolyzing enzymes. Based on the results, these enzymes were classified into four classes.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
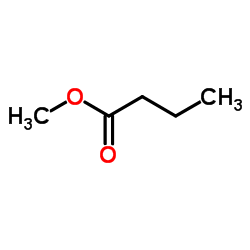 |
Methyl butyrate
CAS:623-42-7 |
C5H10O2 |
|
Combined effects of nutrients and temperature on the product...
2015-03-01 [Appl. Microbiol. Biotechnol. 99(5) , 2291-304, (2015)] |
|
Mosquito odorant receptor for DEET and methyl jasmonate.
2014-11-18 [Proc. Natl. Acad. Sci. U. S. A. 111(46) , 16592-7, (2014)] |
|
An in vitro evaluation of the effect of probiotics and prebi...
2012-08-01 [Anaerobe 18(4) , 386-91, (2012)] |
|
Gene Cloning, High-Level Expression, and Characterization of...
2015-06-01 [J. Microbiol. Biotechnol. 25 , 845-55, (2015)] |
|
Chemical Kinetic Influences of Alkyl Chain Structure on the ...
2015-07-16 [J. Phys. Chem. A 119 , 7559-77, (2015)] |