Molecular modeling of wild-type and antifolate resistant mutant Plasmodium falciparum DHFR.
Reinaldo Teixeira Delfino, Osvaldo Andrade Santos-Filho, José Daniel Figueroa-Villar
Index: Biophys. Chem. 98(3) , 287-300, (2002)
Full Text: HTML
Abstract
The development of drug resistance is reducing the efficiency of antifolates as antimalarials. This phenomenon has been linked to the occurrence of mutations in the parasite's dihydrofolate reductase (DHFR). In this way, the resistance to pyrimethamine and cycloguanil, two potent inhibitors of P. falciparum DHFR, is mainly related to mutations (single and crossed) at residues 16, 51, 59, 108 and 164 of the enzyme. In this work, we have refined a recently proposed homology-model of P. falciparum DHFR, and the resulting structure was used to obtain models for 14 mutant enzymes, employing molecular modeling. Ternary complexes of the mutant enzymes with these inhibitors have been superimposed to equivalent ternary complexes of the wild-type enzyme, allowing the proposition of hypotheses for the role of each mutation in drug resistance. Based on these results, possible reasons for antifolate resistance have been proposed.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
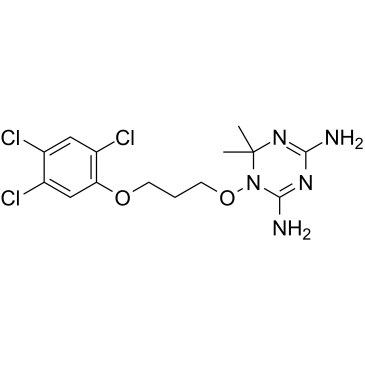 |
WR99210
CAS:47326-86-3 |
C14H18Cl3N5O2 |
|
Development of a genetic tool for functional screening of an...
2015-01-01 [Malaria Journal 14 , 233, (2015)] |
|
Functional analysis of Plasmodium vivax dihydrofolate reduct...
2012-01-01 [PLoS ONE 7(7) , e40416, (2012)] |
|
Novel Saccharomyces cerevisiae screen identifies WR99210 ana...
2002-11-01 [Antimicrob. Agents Chemother. 46(11) , 3362-9, (2002)] |
|
Identification and analysis of dihydrofolate reductase allel...
1999-07-01 [Am. J. Trop. Med. Hyg. 61(1) , 131-40, (1999)] |
|
Novel alleles of the Plasmodium falciparum dhfr highly resis...
2001-09-28 [Mol. Biochem. Parasitol. 117(1) , 91-102, (2001)] |