NK cells in the CD19- B220+ bone marrow fraction are increased in senescence and reduce E2A and surrogate light chain proteins in B cell precursors.
Anne M King, Patricia Keating, Anjali Prabhu, Bonnie B Blomberg, Richard L Riley
Index: Mech. Ageing Dev. 130(6) , 384-92, (2009)
Full Text: HTML
Abstract
E2A encoded proteins, key transcriptional regulators in B lineage specification and commitment, have been shown to decrease in B cell precursors in old age. E2A regulates genes encoding the surrogate light chain proteins lambda5 and VpreB. In old age, B cell precursors express less surrogate light chain and this results in compromised pre-B cell receptor function and diminished expansion of new pre-B cells in senescence. Herein, we show that aged bone marrow has increased Hardy Fraction A (CD19(-) B220(+)) cells, including NK cells, that can inhibit both E47 (E2A) protein and surrogate light chain protein expression in B cell precursors. In vitro, NK-associated inhibition of E47 protein is contact-independent and partially reversed by neutralization of TNFalpha. In vivo, depletion of NK cells in aged mice by treatment with anti-asialo GM1 antibody led to restoration of surrogate light chain protein levels to that typical of young B cell precursors. These studies suggest that NK cells, within the CD19(-) B220(+) bone marrow cell fraction, may contribute to a bone marrow microenvironment that has the potential to negatively regulate E47 (E2A) as well as surrogate light chain levels in B cell precursors in old age.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
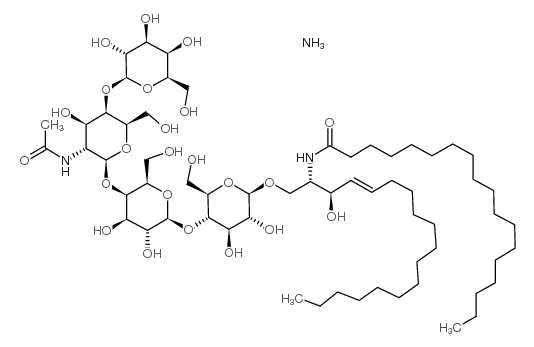 |
Ganglioside GM1, Asialo
CAS:71012-19-6 |
C62H117N3O23 |
|
Fuzheng Huayu inhibits carbon tetrachloride-induced liver fi...
2013-01-09 [J. Ethnopharmacol. 145(1) , 175-81, (2013)] |
|
Sex-dependent liver colonization of human melanoma in SCID m...
2013-04-01 [Clin. Exp. Metastasis 30(4) , 497-506, (2013)] |
|
T lymphocytes restrain spontaneous metastases in permanent d...
2014-04-01 [Cancer Res. 74(7) , 1958-68, (2014)] |
|
Segregative clustering of Lo and Ld membrane microdomains in...
2012-11-27 [Langmuir 28(47) , 16327-37, (2012)] |
|
NK depletion results in increased CCL22 secretion and Treg l...
2010-12-01 [Int. J. Cancer 127(11) , 2598-611, (2010)] |