| Structure | Name/CAS No. | Articles |
|---|---|---|
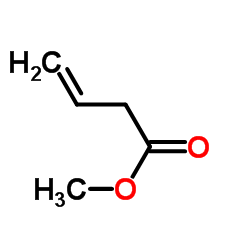 |
Methyl 3-butenoate
CAS:3724-55-8 |
|
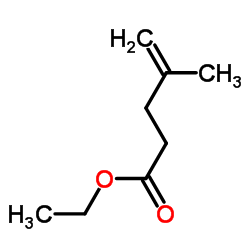 |
Ethyl 4-methyl-4-pentenoate
CAS:4911-54-0 |