Enzymatic synthesis of 2'-deoxyguanosine with nucleoside deoxyribosyltransferase-II.
Kiyoshi Okuyama, Susumu Shibuya, Tomoki Hamamoto, Toshitada Noguchi
Index: Biosci. Biotechnol. Biochem. 67 , 989-95, (2003)
Full Text: HTML
Abstract
Nucleoside deoxyribosyltransferase-II (NdRT-II) of Lactobacillus helveticus, which catalyzes the transfer of a glycosyl residue from a donor deoxyribonucleoside to an acceptor base, has a broad specificity for the acceptor bases. Six-substituted purines were found to be substrates as acceptor bases for NdRT-II. Using this property of the enzyme, we established a practical procedure for enzymatic synthesis of 2'-deoxyguanosine (dGuo), consisting of the transglycosylation from thymidine to 6-substituted purine (2-amino-6-chloropurine; ACP) instead of natural guanine and the conversion of 2-amino-6-chloropurine-2'-deoxyriboside (ACPdR) to dGuo with bacterial adenosine deaminase. Through the successive reactions, dGuo was synthesized in high yield.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
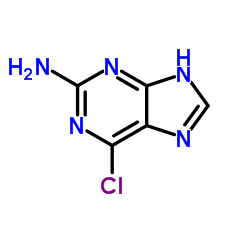 |
2-Amino-6-chloropurine
CAS:10310-21-1 |
C5H4ClN5 |
|
The screening and characterization of 6-aminopurine-based xa...
2007-05-15 [Bioorg. Med. Chem. 15 , 3450-6, (2007)] |
|
Molecular crowding enhances facilitated diffusion of two hum...
2015-04-30 [Nucleic Acids Res. 43 , 4087-97, (2015)] |
|
Additive Pummerer reaction of 3,5-O-(di-tert-butyl)silylene-...
2009-03-20 [J. Org. Chem. 74(6) , 2616-9, (2009)] |
|
Testing nucleoside analogues as inhibitors of Bacillus anthr...
2010-12-01 [Antimicrob. Agents Chemother. 54 , 5329-36, (2010)] |
|
Lead compounds for antimalarial chemotherapy: purine base an...
2006-12-14 [J. Med. Chem. 49 , 7479-86, (2006)] |