Improved separation of diastereomeric derivatives of enantiomers by a physical network of linear polyvinylpyrrolidone applied as pseudophase in capillary zone electrophoresis.
W Schützner, G Caponecchi, S Fanali, A Rizzi, E Kenndler
Index: Electrophoresis 15(6) , 769-73, (1994)
Full Text: HTML
Abstract
Diastereomeric analytes were separated using capillary zone electrophoresis with polyvinylpyrrolidone as polymeric additive to the buffer solution. As test substances derivatives of D- and L- alpha-amino and alpha-hydroxy acids formed by reaction with (+)-O,O'-diacetyl- and (+)-O,O'-dibenzoyl-L-tartaric anhydride, respectively, were used. The physical network formed by the linear polymer is supposed to act as a kind of pseudophase. It was found that the network affects the mobility of diastereomeric compounds to a different extent, enhancing the selectivity of the system. In nearly all cases of aromatic acids the diastereomer carrying the D-amino acid was more strongly retained than the L-isomer, as opposed to the situation with aliphatic acids.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
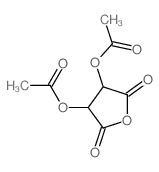 |
(+)-DIACETYL-L-TARTARIC ANHYDRIDE
CAS:6283-74-5 |
C8H8O7 |
|
Enantioselective drug monitoring of (R)- and (S)- propranolo...
[J. Chromatogr. A. 487 , 375, (1989)] |
|
Analytical and semi-preparative high-performance liquid chro...
[J. Chromatogr. A. 581(1) , 83-92, (1992)] |
|
Measurement of urinary D- and L-2-hydroxyglutarate enantiome...
2004-08-01 [Clin. Chem. 50(8) , 1391-5, (2004)] |
|
2-hydroxyglutarate production, but not dominant negative fun...
2011-01-01 [PLoS ONE 6 , e16812, (2011)] |
|
A liquid chromatographic assay for the stereospecific quanti...
1995-06-01 [J. Pharm. Biomed. Anal. 13(7) , 911-8, (1995)] |