| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
(E,Z)-3,7,11-Trimethyl-2,6,10-dodecatrien-1-ol
CAS:3879-60-5 |
|
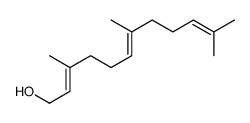 |
(Z,E)-farnesol
CAS:3790-71-4 |
|
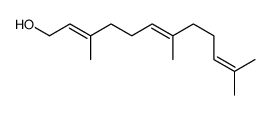 |
2Z,6Z-Farnesol
CAS:16106-95-9 |