Immunomodulation activity of phenothiazines, benzo[a]phenothiazines and benz[c]acridines.
J Molnar, Y Mandi, I Petri, S Petofi, H Sakagami, T Kurihara, N Motohashi
Index: Anticancer Res. 13(2) , 439-42, (1993)
Full Text: HTML
Abstract
Some non-differentiation-induction benzo[a]phenothiazines and mutagenic benz[c]acridines more potently inhibited the mitogen-induced blast transformation of human-peripheral blood lymphocytes than differentiation-induction and non-mutagenic counterparts and phenothiazines. Differential absorption spectrophotometry revealed tight complex formation between these drugs and bacterial endotoxin or mitogens. All of these compounds only slightly affected antibody dependent cellular cytotoxicity and natural killer cell activity, but significantly inhibited the endotoxin-or heat-killed Staphylococcus aureus induced tumor necrosis factor production by human mononuclear cells. Pretreatment of mice with these drugs protected them from lethal E. coli infection. Quantumchemical analysis suggests a correlation between the biological activity of these compounds and some molecular orbital parameters such as the charge at C7, and the ratio of polar/total surface areas.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
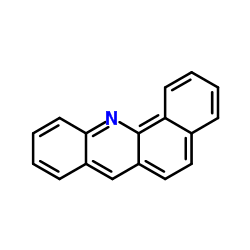 |
BENZ(C)ACRIDINE
CAS:225-51-4 |
C17H11N |
|
Substituted benz[a]acridines and benz[c]acridines as mammali...
2000-05-01 [Bioorg. Med. Chem. 8(5) , 1171-82, (2000)] |
|
Heteroatom effects in chemical carcinogenesis: effects of ri...
1983-12-01 [Cancer Biochem. Biophys. 7(1) , 53-60, (1983)] |
|
Differentiated genotoxic response of carcinogenic and non-ca...
1985-03-01 [Carcinogenesis 6(3) , 455-7, (1985)] |
|
Surface changes and hormone production in pituitary cells du...
1999-12-01 [J. Exp. Clin. Cancer Res 18(4) , 553-8, (1999)] |
|
Antiplasmid and carcinogenic molecular orbitals of benz[c]ac...
1993-01-01 [Anticancer Res. 13(1) , 263-6, (1993)] |