| Structure | Name/CAS No. | Articles |
|---|---|---|
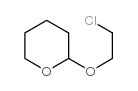 |
2H-Pyran,2-(2-chloroethoxy)tetrahydro
CAS:5631-96-9 |
|
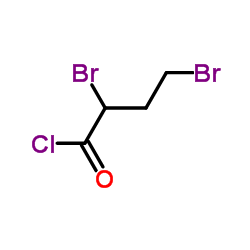 |
2,4-Dibromobutanoyl chloride
CAS:82820-87-9 |