The role of dietary ergothioneine in the development of diabetes mellitus.
R M Epand
Index: Med. Hypotheses 9(2) , 207-13, (1982)
Full Text: HTML
Abstract
Ergothioneine is believed not to be synthesized by man but it accumulates to high concentrations in some mammalian cells as a result of dietary intake. Ergothioneine is known to chelate divalent metal ions with high affinity. Other substances which are potent chelators of divalent metal ions such as diphenylthiocarbazone and quinaldic acid are known to be potent diabetogenic agents. It is therefore likely that because of its high concentration in man and its high affinity for divalent cations, ergothioneine is a naturally occurring chelating agent which can be a contributing factor leading to the development of diabetes mellitus in some individuals. In one study it has been shown that some diabetic patients have markedly elevated levels of ergothioneine compared with normal individuals. The mechanism by which ergothioneine induces diabetes may be through its chelation of zinc which is important for the storage of insulin and glucagon.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
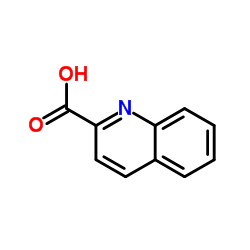 |
Quinoline-2-carboxylic acid
CAS:93-10-7 |
C10H7NO2 |
|
Biosynthesis of 8-hydroxyquinoline-2-carboxylic acid, an iro...
2015-01-07 [Org. Biomol. Chem. 13(1) , 178-84, (2014)] |
|
Hydrogen-bonded frameworks of bis(2-carboxypyridinium) hexaf...
2007-09-01 [Acta Crystallogr. C 63(Pt 9) , o530-4, (2007)] |
|
How thiostrepton was made in the laboratory.
2012-12-07 [Angew. Chem. Int. Ed. Engl. 51(50) , 12414-36, (2012)] |
|
Insights into quinaldic acid moiety formation in thiostrepto...
2012-04-20 [Chem. Biol. 19(4) , 443-8, (2012)] |
|
Synthesis, characterization and crystal structure of novel m...
2009-05-01 [J. Inorg. Biochem. 103(5) , 859-68, (2009)] |