Transcriptional response pathways in a yeast strain sensitive to saframycin a and a more potent analog: evidence for a common basis of activity.
Alleyn T Plowright, Scott E Schaus, Andrew G Myers
Index: Chem. Biol. 9(5) , 607-18, (2002)
Full Text: HTML
Abstract
Saframycin A (SafA) is a natural product that inhibits human cancer cell proliferation. Its synthetic analog, QAD, is a more potent inhibitor of these cells. SafA does not affect wild-type yeast, but it does inhibit growth of the strain CCY333 (DeltaPDR1/PDR3/ERG6) (IC50 = 0.9 microM). QAD is also a more effective inhibitor of CCY333 growth (IC50 = 0.4 microM). Transcription profiling of SafA- and QAD-treated CCY333 cultures showed that both drugs generated nearly identical profiles, with altered expression levels (> or =2-fold) of more than 240 genes. Both agents induced the overexpression of genes involved in glycolysis, oxidative stress, and protein degradation and repressed genes encoding histones, biosynthetic enzymes, and the cellular import machinery. Significantly, neither drug affected the expression of known DNA-damage repair genes, as might have been expected if their primary mechanism of action involved the covalent modification of DNA.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
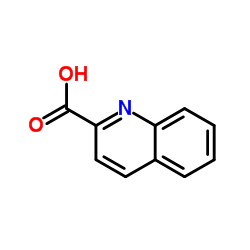 |
Quinoline-2-carboxylic acid
CAS:93-10-7 |
C10H7NO2 |
|
Biosynthesis of 8-hydroxyquinoline-2-carboxylic acid, an iro...
2015-01-07 [Org. Biomol. Chem. 13(1) , 178-84, (2014)] |
|
Hydrogen-bonded frameworks of bis(2-carboxypyridinium) hexaf...
2007-09-01 [Acta Crystallogr. C 63(Pt 9) , o530-4, (2007)] |
|
How thiostrepton was made in the laboratory.
2012-12-07 [Angew. Chem. Int. Ed. Engl. 51(50) , 12414-36, (2012)] |
|
Insights into quinaldic acid moiety formation in thiostrepto...
2012-04-20 [Chem. Biol. 19(4) , 443-8, (2012)] |
|
Synthesis, characterization and crystal structure of novel m...
2009-05-01 [J. Inorg. Biochem. 103(5) , 859-68, (2009)] |