Inhibition of dopamine beta-hydroxylase by bidentate chelating agents.
S Townes, C Titone, R C Rosenberg
Index: Biochim. Biophys. Acta 1037(2) , 240-7, (1990)
Full Text: HTML
Abstract
1-2H-Phthalazine hydrazone (hydralazine; HYD), 2-1H-pyridinone hydrazone (2-hydrazinopyridine; HP), 2-quinoline-carboxylic acid (QCA), 1-isoquinolinecarboxylic acid (IQCA), 2,2'-bi-1H-imidazole (2,2'-biimidazole; BI), and 1H-imidazole-4-acetic acid (imidazole-4-acetic acid; IAA) directly and reversibly inhibit homogeneous soluble bovine dopamine beta-hydroxylase (3,4-dihydroxyphenethylamine, ascorbate:oxygen oxidoreductase (beta-hydroxylating), EC 1.14.17.1). HYD, QCA and IAA show competitive allosteric inhibition of dopamine beta-hydroxylase with respect to ascorbate (Kis = 5.7(+/- 0.9) microM, 0.14(+/- 0.03) mM, 0.80(+/- 0.20) mM; nH = 1.4(+/- 0.1), 1.8(+/- 0.4), 2.8(+/- 0.6), respectively). HYD and IAA show slope and intercept mixed-type allosteric inhibition of dopamine beta-hydroxylase with respect to tyramine. QCA shows allosteric uncompetitive inhibition of dopamine beta-hydroxylase with respect to tyramine. HP, BI and IQCA all show linear competitive inhibition (Kis = 1.9(+/- 0.3) microM, 21(+/- 6) microM, and 0.9(+/- 0.3) microM, respectively) with respect to ascorbate. HP and BI show linear mixed-type while IQCA shows linear uncompetitive inhibition of dopamine beta-hydroxylase with respect to tyramine. In the presence of HP, HYD or IAA intersecting double-reciprocal plots of the initial velocity as a function of tyramine concentration at differing fixed levels of ascorbate are observed. These findings are consistent with a uni-uni-ping-pong-ter-bi kinetic mechanism for dopamine beta-hydroxylase that involves a ternary enzyme-ascorbate-tyramine-oxygen complex. The results for HYD, QCA and IAA are the first examples of allosteric inhibitor interactions with dopamine beta-hydroxylase.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
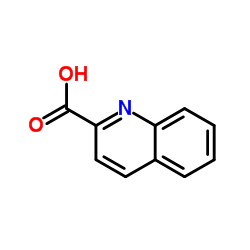 |
Quinoline-2-carboxylic acid
CAS:93-10-7 |
C10H7NO2 |
|
Biosynthesis of 8-hydroxyquinoline-2-carboxylic acid, an iro...
2015-01-07 [Org. Biomol. Chem. 13(1) , 178-84, (2014)] |
|
Hydrogen-bonded frameworks of bis(2-carboxypyridinium) hexaf...
2007-09-01 [Acta Crystallogr. C 63(Pt 9) , o530-4, (2007)] |
|
How thiostrepton was made in the laboratory.
2012-12-07 [Angew. Chem. Int. Ed. Engl. 51(50) , 12414-36, (2012)] |
|
Insights into quinaldic acid moiety formation in thiostrepto...
2012-04-20 [Chem. Biol. 19(4) , 443-8, (2012)] |
|
Synthesis, characterization and crystal structure of novel m...
2009-05-01 [J. Inorg. Biochem. 103(5) , 859-68, (2009)] |