Hemodynamic effects of once-daily tadalafil in men with signs and symptoms of benign prostatic hyperplasia on concomitant α1-adrenergic antagonist therapy: results of a multicenter randomized, double-blind, placebo-controlled trial.
Evan Goldfischer, John J Kowalczyk, William R Clark, Erin Brady, Michael Anne Shane, Nancy Dgetluck, Suzanne R Klise
Index: Urology 79(4) , 875-82, (2012)
Full Text: HTML
Abstract
To investigate the safety of daily coadministration of α-blockers with tadalafil 5 mg in men with lower urinary tract symptoms secondary to benign prostatic hyperplasia. The standard-of-care medical therapy for moderate to severe symptoms of benign prostatic hyperplasia is α(1)-adrenergic antagonist (α-blocker) therapy.Men aged ≥ 45 years receiving stable α-blocker therapy were evaluated for eligibility before a 2-week single-blind, placebo lead-in period. Subsequently, 318 men were randomized to tadalafil 5 mg or placebo once daily for 12 weeks. Enrollment was monitored to ensure inclusion of men ≥ 75 years old and men taking nonuroselective α-blockers. The primary objective was to compare the proportion of men reporting treatment-emergent dizziness between the 2 treatment groups. Orthostatic vital signs, general safety, and the International Prostate Symptom Score were also assessed.The proportion of patients who reported treatment-emergent dizziness was not significantly different between the 2 treatment groups (tadalafil 7.0%; placebo 5.7%; P = .403). No difference between treatment groups was observed with respect to patients meeting the criteria for a positive orthostatic test (30 per treatment group, P = 1.00). The incidence of discontinuations was low among both treatment groups.Recognizing the limitations of the present study, the changes in the hemodynamic signs and symptoms were similar for the tadalafil and placebo groups in men with benign prostatic hyperplasia receiving concomitant α-blocker therapy. However, consistent with the results of previous clinical pharmacology studies of healthy subjects, a trend was seen for increased hemodynamic signs and symptoms in men taking nonuroselective α-blockers, most notably those taking doxazosin.Copyright © 2012 Elsevier Inc. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
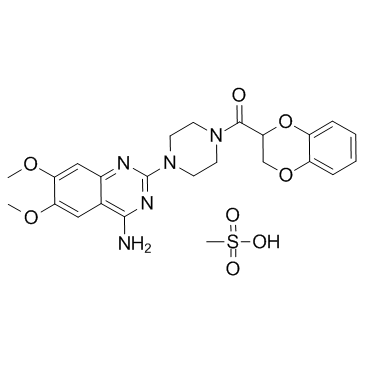 |
Doxazosin mesylate
CAS:77883-43-3 |
C24H29N5O8S |
|
Systems pharmacology identifies drug targets for Stargardt d...
2013-12-01 [J. Clin. Invest. 123(12) , 5119-34, (2013)] |
|
The efficacy and safety of alpha-1 blockers for benign prost...
2013-03-01 [Curr. Med. Res. Opin. 29(3) , 279-87, (2013)] |
|
[Results of open multicenter study of the safety of doxazosi...
2013-01-01 [Urologiia. (2) , 42-4, 46, (2013)] |
|
Irbesartan, an FDA approved drug for hypertension and diabet...
2015-08-01 [Antiviral Res. 120 , 140-6, (2015)] |
|
Long-term effects of doxazosin, finasteride and combination ...
2013-07-01 [J. Urol. 190(1) , 187-93, (2013)] |