N-linked oligosaccharide structures in the diamine oxidase from porcine kidney.
Y Huang, Y Mechref, M V Novotny
Index: Carbohydr. Res. 323 , 111-25, (2000)
Full Text: HTML
Abstract
Structures of the N-linked glycans released from porcine kidney diamine oxidase (DAO) were characterized utilizing various analytical techniques, including matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI/TOF-MS), high-performance capillary electrophoresis (HPCE), and high-pH anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD). The oligosaccharide sequences present in DAO were conclusively determined using specific exoglycosidases in conjunction with MALDI/TOF-MS. The structures found in the glycoprotein are primarily linear, di-, or tribranched fucosylated complex type. MS analysis of the esterified N-glycan pool derived from DAO indicated the presence of several di- and trisialylated structures.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
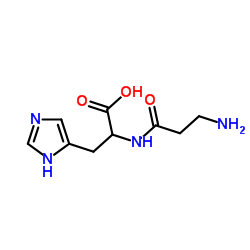 |
Diamine Oxidase from porcine kidney
CAS:9001-53-0 |
C9H14N4O3 |
|
Diamine oxidase from pig kidney: new purification method and...
1982-01-01 [Prep. Biochem. 12(1) , 11-28, (1982)] |
|
The glycoprotein nature of pig kidney diamine oxidase. Role ...
1988-07-01 [Biochem. J. 253(1) , 103-7, (1988)] |
|
A luminescence-based test for determining ornithine decarbox...
2000-12-15 [Anal. Biochem. 287 , 299-302, (2000)] |
|
Diamine oxidase and catalase are expressed in the same cells...
1999-04-01 [Inflamm. Res. 48 , s81-2, (1999)] |
|
Evolution of a constitutional dynamic library driven by self...
2010-04-26 [Chemistry 16(16) , 4903-10, (2010)] |