Recognition of Type 1 Chain Oligosaccharides and Lacto-Series Glycolipids by an Antibody to Human Secretory Component
H. Yu, J.M. Sipes, J. Cashel, M.A. Bakos, R.M. Goldblum, D.D. Roberts, H Yu, J M Sipes, J Cashel, M A Bakos, R M Goldblum, D D Roberts
Index: Arch. Biochem. Biophys. 322(2) , 299-305, (1995)
Full Text: HTML
Abstract
Binding of the mouse IgM antibody 6C4 is lost after treatment of human free secretory component with peptide N-glycosidase F (Bakos et al. (1991) J. Immunol. 146, 162-168) or periodate, suggesting that asparagine-linked oligosaccharides contain the epitope recognized by this antibody. Inhibition of antibody binding to free secretory component by milk oligosaccharides established that lacto-N-tetraose is the minimum structure recognized by the antibody, but larger oligosaccharides with terminal Galβ1-3GlcNAc sequences bind with much higher affinity. Antibody binding is enhanced by substitution with the Lewis Fucα1-4 and is inhibited by Fucα1-2Gal substitution. Free secretory component, however, does not bind other antibodies that recognize Lea or Leb oligosaccharides, and binding is lost after digestion with a β-galactosidase that cleaves Galβ1-3 linkages but not after digestion with α-L-fucosidase. Therefore, the major epitope recognized by 6C4 on free secretory component is probably not an asparagine-linked Lea oligosaccharide. The antibody also binds to human milk lactoferrin, some human mucins, and lacto-series glycolipids including III4αFuc-lactotetraosyl ceramide and lactotetraosyl ceramide. Based on affinity chromatography of oligosaccharides released from free secretory component, the epitope recognized by antibody 6C4 is present on approximately 3.5% of the asparagine-linked oligosaccharides.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
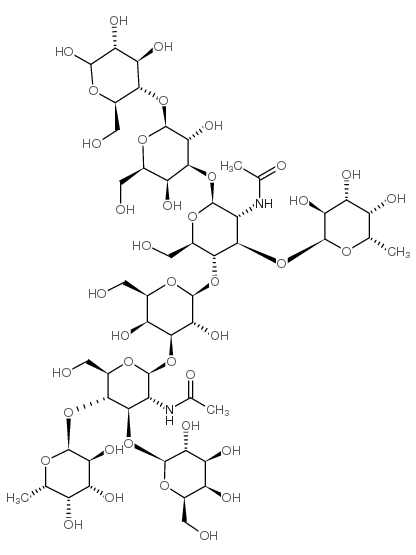 |
Difucosyl-para-lacto-N-hexaose II (DFpLNH II)
CAS:64309-01-9 |
C52H88N2O39 |
|
Glycoprofiling of bifidobacterial consumption of human milk ...
2007-10-31 [J. Agric. Food Chem. 55(22) , 8914-9, (2007)] |
|
The role of gut-associated lymphoid tissues and mucosal defe...
2005-04-01 [Br. J. Nutr. 93 Suppl 1 , S41-8, (2005)] |
|
Fucose-containing oligosaccharides from human milk from a do...
1988-04-01 [Biol. Chem. Hoppe-Seyler 369(4) , 257-73, (1988)] |
|
Primary structure of four human milk octa-, nona-, and undec...
1992-03-16 [Carbohydr. Res. 226(1) , 1-14, (1992)] |
|
Total syntheses of tumor-related antigens N3: probing the fe...
2001-01-10 [J. Am. Chem. Soc. 123(1) , 35-48, (2001)] |