Si(IV)-naphthalocyanine: modulation of its pharmacokinetic properties through the use of hydrophilic axial ligands.
C Bellemo, G Jori, B D Rihter, M E Kenney, M A Rodgers
Index: Cancer Lett. 65(2) , 145-50, (1992)
Full Text: HTML
Abstract
A water-soluble derivative of the highly hydrophobic molecule Si(IV)-naphthalocyanine was synthesized by the addition of two polyethyleneglycol ligands in axial positions to the centrally coordinated Si(IV) ion. The compound can be intravenously injected in homogeneous aqueous solution, where it is largely aggregated and remains mostly in an unbound state. While the naphthalocyanine is accumulated in significant amounts by an intramuscularly transplanted MS-2 fibrosarcoma in Balb/c mice, the dye shows little tumour selectivity and essentially no phototherapeutic activity. The situation is not improved by association of the naphthalocyanine with dipalmitoyl-phosphatidylcholine liposomes, probably owing to a lack of incorporation of the bulky dye molecule into the phospholipid bilayer.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
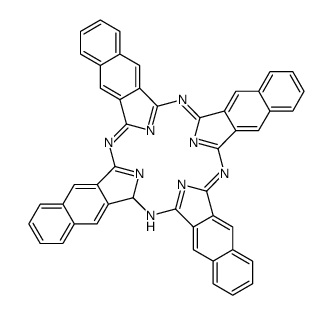 |
2,3-naphthalocyanine
CAS:23627-89-6 |
C48H26N8 |
|
Recent advances in submolecular resolution with scanning pro...
2011-04-01 [Nature Chemistry 3(4) , 273-8, (2011)] |
|
Soluble precursors of 2,3-naphthalocyanine and phthalocyanin...
2008-10-21 [Chem. Commun. (Camb.) (39) , 4714-6, (2008)] |
|
Spatial imaging of individual vibronic states in the interio...
2011-07-07 [J. Chem. Phys. 135(1) , 014705, (2011)] |
|
Tetraamido-substituted 2,3-naphthalocyanine zinc(II) complex...
1996-09-01 [J. Photochem. Photobiol. B, Biol. 35(3) , 167-74, (1996)] |
|
Photoinactivation of amelanotic and melanotic melanoma cells...
1998-03-01 [J. Photochem. Photobiol. B, Biol. 42(3) , 202-10, (1998)] |