Orange peel as an adsorbent in the removal of acid violet 17 (acid dye) from aqueous solutions.
R Sivaraj, C Namasivayam, K Kadirvelu
Index: Waste Manag. 21(1) , 105-10, (2001)
Full Text: HTML
Abstract
The effectiveness of orange peel in adsorbing Acid violet 17 from aqueous solutions has been studied as a function of agitation time, adsorbent dosage, initial dye concentration and pH. The adsorption follows both Langmuir and Freundlich isotherms. The adsorption capacity Q0 was 19.88 mg/g at initial pH 6.3. The equilibrium time was found to be 80 min for 10, 20, 30 and 40 mg/L, dye concentration respectively. A maximum removal of 87% was obtained at pH 2.0 for an adsorbent dose of 600 mg/50 ml of 10 mg/L dye concentration. Adsorption increases with increase in pH. Maximum desorption of 60% was achieved in water medium at pH 10.0.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
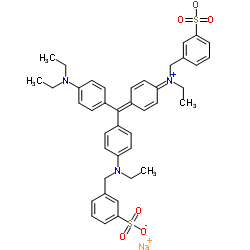 |
Acid violet 17
CAS:4129-84-4 |
C41H44N3NaO6S2 |
|
An investigation into the enhancement of fingermarks in bloo...
2013-09-01 [Sci. Justice 53(3) , 321-7, (2013)] |
|
Comparative mutagenicity of two triarylmethane food dyes in ...
1981-08-01 [Food Cosmet. Toxicol. 19(4) , 419-24, (1981)] |
|
[Development and clinical evaluation of a new urinary protei...
1991-04-01 [Rinsho Byori. 39(4) , 398-404, (1991)] |
|
Removal of Acid Violet 17 from aqueous solutions by adsorpti...
2008-03-01 [J. Hazard. Mater. 151(2-3) , 316-22, (2008)] |
|
Bioaccumulation of the synthetic dye Basic Violet 3 and heav...
2011-02-28 [J. Hazard. Mater. 186(2-3) , 1541-52, (2011)] |