[Phenoxenium ions: generations and reactions].
Y Endo, K Shudo
Index: Yakugaku Zasshi 114(8) , 565-76, (1994)
Full Text: HTML
Abstract
The acid-catalyzed reaction of N-acyl- and N-sulfonylhydroxylamines with benzene proceeded smoothly to give C-C products; 2- and 4-hydroxybiphenyls. The reaction and the thermolysis of N-aryloxypyridinium salts involve common intermediates. The results of product analysis, the orientation of the reaction, effects of substituents on the nitrogen atom and on the phenyl ring suggested a mechanism involving a phenoxenium ion. The positive charge of the phenoxenium ion localized not on the oxygen atom but on the ortho and para carbons of the benzene ring. C-O product: diphenylethers are formed when the heterolysis of the N-O bonds is slow and the aromatic solvent has high nucleophilicity, suggesting an SN2-like reaction on the oxygen atom. The phenoxenium ions are also concerned with a rearrangement of O-arylhydroxylamine to 2-aminophenol. An ion-molecule pair involving phenoxenium ion and ammonia as an intermediate of the intramolecular ortho rearrangement.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
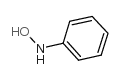 |
N-Phenylhydroxylamine
CAS:100-65-2 |
C6H7NO |
|
In situ infrared monitoring of the solid/liquid catalyst int...
2011-07-21 [Phys. Chem. Chem. Phys. 13(27) , 12463-71, (2011)] |
|
Genotoxic activities of aniline and its metabolites and thei...
2005-12-01 [Crit. Rev. Toxicol. 35(10) , 783-835, (2005)] |
|
Biotransformation of hydroxylaminobenzene and aminophenol by...
2000-06-01 [Appl. Environ. Microbiol. 66(6) , 2336-42, (2000)] |
|
Superoxide dismutase-mediated reversible conversion of 3-hyd...
1988-11-01 [Carcinogenesis 9(11) , 2003-8, (1988)] |
|
Thrombelastographic characterization of coagulation/fibrinol...
2013-04-01 [Blood Coagul. Fibrinolysis 24(3) , 273-8, (2013)] |