| Structure | Name/CAS No. | Articles |
|---|---|---|
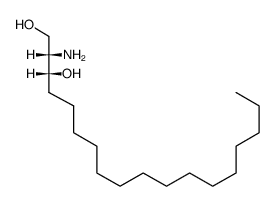 |
DL-THREO-DIHYDROSPHINGOSINE
CAS:73938-69-9 |
| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
DL-THREO-DIHYDROSPHINGOSINE
CAS:73938-69-9 |